Clinical Translation Start Trials - ClinicStarT
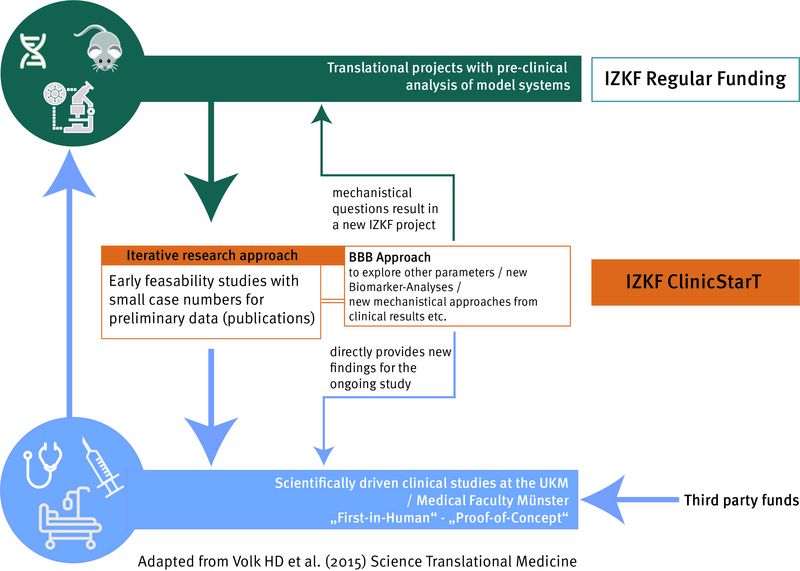
In the framework of promoting translational research, the IZKF Münster has developed innovative concepts to close the gap between funding basic research (e.g. mouse studies) and patient-centred research.
Since 2019, the "ClinicStarT" programme funds innovative concepts and science-driven ideas for clinical studies that will later be transferred to external funding programmes. These include clinical observation studies, early clinical feasibility studies (proof of concept) and first pilot studies on patients, which should bring knowledge from basic research and animal experiments into clinical trials.


