Research focus
Evolutionary adaptations of spermatogenesis and testicular structures – spermatogonial stem cell systems, reproductive endocrinology and sperm competition
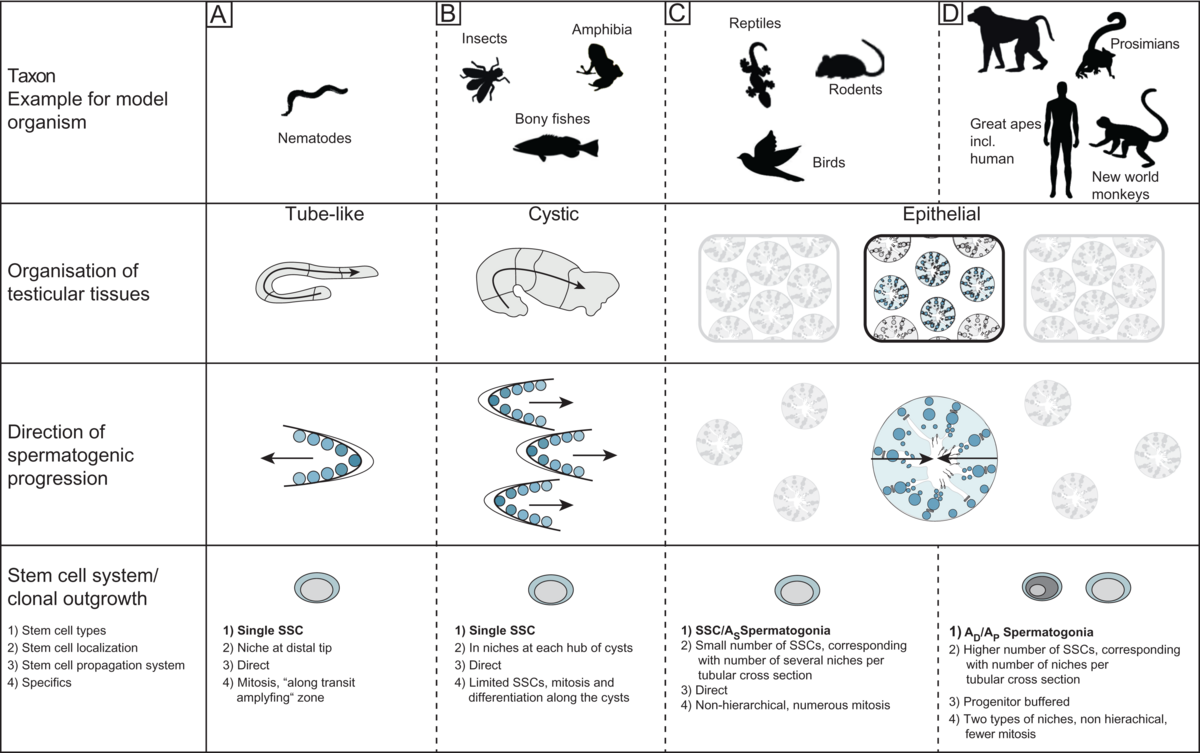
Comparison of testicular organizational structures in model organisms (Sharma et al. 2019) Male reproduction is challenged by evolution with regard to the formation of „successful” gametes. Amongst these challenges two are most relevant - sperm competition and spermatogonial stem cell (SSC) systems.While the concept of sperm competition is generally accepted only little is known whether and if by which mechanisms it is reflected in spermatogenesis as such (Ramm et al. 2014). In principle, three possible mechanisms (or a combination of those) can be suggested: For the sperm, a successful fertilization of the egg occurs when it has won the race. For this purpose, the progressive motility of sperm is crucial, which depends on the number of mitochondria, and the performance of the flagellum, i.e. the length of the sperm. Furthermore, the sheer number of available sperm is a logical determinant which is correlated with the amount of seminiferous epithelium and / or with the rate of sperm production (cycle length).We are interested in the question how sperm competition in testes of primates and birds and have been able to show that there are different adaptations to this challenge in these taxa. While competitive conditions are actually reflected in primates only in relative testes size, birds adjusted the ratio of seminiferous to interstitial tissue and produced sperm of different lengths. Preliminary data also indicate that the duration for the production of mature gametes (spermatogenic cycle length) can vary. The germ line function should also be adjusted for short or long reproductive periods during life. For example, a mouse reproduces only during 1.5 years; monkeys, however, are for fertile many years, increasing the risk of injury or infection. This means that the mission of providing the highest possible number of fertile gametes has to be balanced against maintaining an intact germ line over time (Sharma et al. 2019). One of the relevant adaptations are different SSC systems, which can be direct (mouse) or progenitor buffered (primates). Our comparative analysis of the organization of the testicular tissue and the endocrine regulation of male reproduction in primates revealed so far unknown findings: On the cellular level and concerning developmental processes, the testicular tissue of New World monkeys corresponds to that of Great Apes (including humans), but differs from Prosimians and Old World monkeys. This could be due to a clonal size and synchrony of SSC derivatives. In contrast, the reproductive endocrine system is very different in New World monkeys from other primates, as luteinizing hormone (LH) is missing and its receptor is mutated, here the Old World monkeys more closely resemble the human situation, via an identical LH / CG - LH receptor system. Thus New World monkeys are a model for research on cellular composition and development of the testis, Old World monkeys, however, should be considered when analyzing hormonal regulation (Luetjens et al 2005, Ehmcke et al. 2006, Wistuba et al. 2007, Wistuba et al. 2013)
Literature:
Ehmcke J, Hübner K, Schöler HR, Schlatt S. Spermatogonia: origin, physiology and prospects for conservation and manipulation of the male germ line. Reprod Fertil Dev. 2006;18(1-2):7-12. doi.org/10.1071/rd05119Gromoll J, Wistuba J, Terwort N, Godmann M, Müller T, Simoni M. A new subclass of the luteinizing hormone/chorionic gonadotropin receptor lacking exon 10 messenger RNA in the New World monkey (Platyrrhini) lineage. Biol Reprod. 2003 Jul;69(1):75-80. doi.org/10.1095/biolreprod.102.014902
Luetjens CM, Weinbauer GF, Wistuba J. Primate spermatogenesis: new insights into comparative testicular organisation, spermatogenic efficiency and endocrine control. Biol Rev Camb Philos Soc. 2005 Aug;80(3):475-88. doi.org/10.1017/s1464793105006755
Lüpold S, Wistuba J, Damm OS, Rivers JW, Birkhead TR. Sperm competition leads to functional adaptations in avian testes to maximize sperm quantity and quality. Reproduction. 2011 May;141(5):595-605. doi.org/10.1530/REP-10-0501
Ramm SA, Schärer L, Ehmcke J, Wistuba J. Sperm competition and the evolution of spermatogenesis. Mol Hum Reprod. 2014 Dec;20(12):1169-79. doi.org/10.1093/molehr/gau070
Sharma S, Wistuba J, Pock T, Schlatt S, Neuhaus N. Spermatogonial stem cells: updates from specification to clinical relevance. Hum Reprod Update. 2019 May 1;25(3):275-297. doi.org/10.1093/humupd/dmz006
Wistuba J, Völker W, Ehmcke J, Clemen G. Characterization of glycosaminoglycans during tooth development and mineralization in the axolotl, Ambystoma mexicanum. Tissue Cell. 2003 Oct;35(5):353-61. doi.org/10.1016/s0040-8166(03)00056-9
Wistuba J, Mittag J, Luetjens CM, Cooper TG, Yeung CH, Nieschlag E, Bauer K. Male congenital hypothyroid Pax8-/- mice are infertile despite adequate treatment with thyroid hormone. J Endocrinol. 2007 Jan;192(1):99-109. doi.org/10.1677/JOE-06-0054
Experimental approaches for translational andrological research
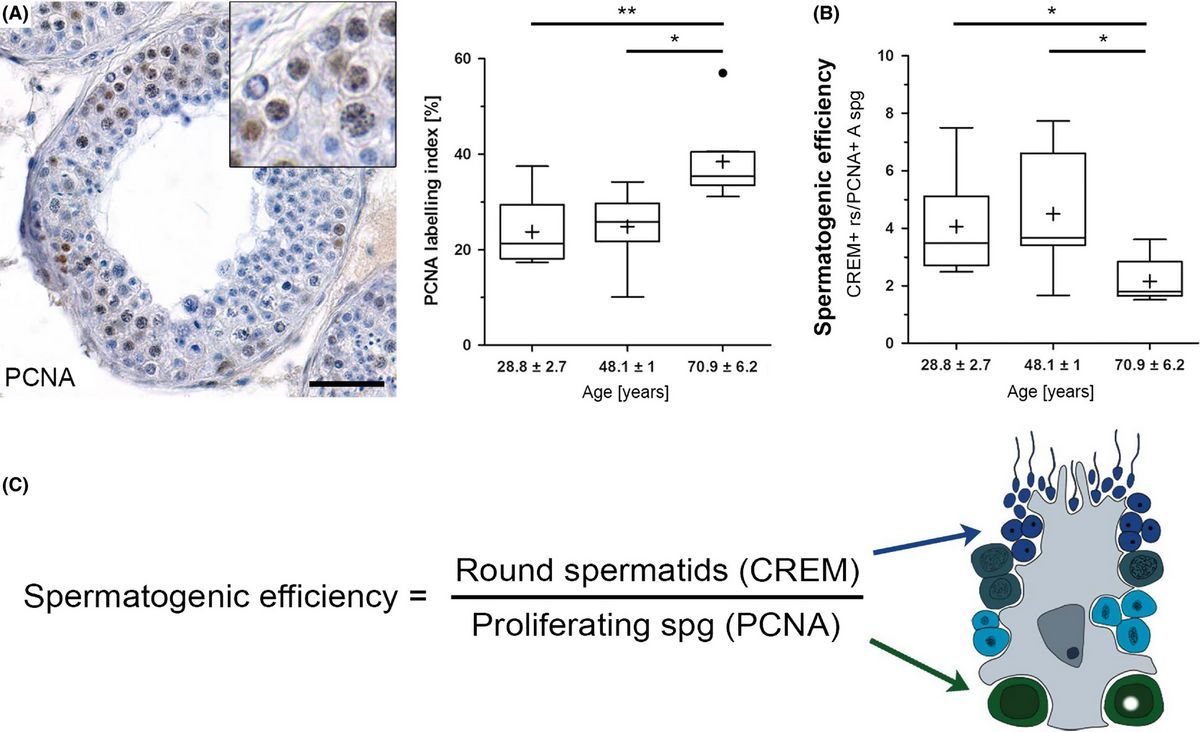
In older men with normal spermatogenesis, the spermatogonial proliferation is increased and the efficiency of the spermatogenesis is decreased. (A) Immunohistochemical staining. (B, C) Efficiency of spermatogenesis and its calculation. (Pohl et al. 2019) A main topic of male reproductive research is fertility and infertility. In recent decades, research has especially focussed on pathophysiologies that cause infertility, as well as on options for providing a reliable male contraception. To understand the physiology of both of these, translational approaches, i.e. animal experimental work, are necessary. Analyzing knockout mouse models, we have studied different gene defects, such as the CREM and Pax8 deficiency and track genetic causes of infertility currently in a mouse model of the Klinefelter syndrome. Another important topic of andrology aims at providing a reliable male contraception on a hormonal basis which we also investigated. We characterized the substances suggested (testosterone esters and gestagens) in their effect on non-human primates, marmosets and macaques (the model of choice for these studies as results from rodent studies are not or only partially transferable), and assessed the NHPs terms of their suitability for such studies. It was an important finding that New World monkeys, due to their specific endocrine regulation, appear inappropriate for studies on hormonal contraception and should not be used (Wistuba et al. 2013). The preservation of the male germ line by transplantation of germ cells or testicular tissue fragments is also a focus of our research. Besides being able to understand the testicular functions better, methods of germ line transplantation also offer options for preclinical developments. We conducted some xenologous and autologous transplantation studies, in which we demonstrated that the transfer of results obtained in rodents is not readily possible to man. Originally developed as a method for the generation of transgenic animals, later adapted also for fertility preservation in child patients suffering from malignancies, the technique might become also an option to maintain male germ lines of endangered species (Luetjens et al 2008, Ntemou et al. 2019, Sharma et al. 2019).
Recently we became interested in changes of testicular physiology and spermatogenesis with regard to ageing. The unique sample collection of testicular biopsies available at CeRA enabled us to alnalyse pure ageing effects and we could show for the first time that the spermatogenic efficiency of the human testis is maintained by an increasing recruitment of reserve Adark spermatogonia which are activated at higher age. Also Sertoli cells undergo age-related changes likely reflecting metabolically altered physiology (Pohl et al. 2016, 2020, 2021).Literature:
Albert S, Ehmcke J, Wistuba J, Eildermann K, Behr R, Schlatt S, Gromoll J. Germ cell dynamics in the testis of the postnatal common marmoset monkey (Callithrix jacchus). Reproduction. 2010 Nov;140(5):733-42. doi.org/10.1530/REP-10-0235
Chandolia RK, Luetjens CM, Wistuba J, Yeung CH, Nieschlag E, Simoni M. Changes in endocrine profile and reproductive organs during puberty in the male marmoset monkey (Callithrix jacchus). Reproduction. 2006 Aug;132(2):355-63. doi.org/10.1530/rep.1.01186
Irfan S, Ehmcke J, Shahab M, Wistuba J, Schlatt S. Immunocytochemical localization of kisspeptin and kisspeptin receptor in the primate testis. J Med Primatol. 2016 Jun;45(3):105-11. doi.org/10.1111/jmp.12212
Junaidi A, Luetjens CM, Wistuba J, Kamischke A, Yeung CH, Simoni M, Nieschlag E. Norethisterone enanthate has neither a direct effect on the testis nor on the epididymis: a study in adult male cynomolgus monkeys (Macaca fascicularis). Eur J Endocrinol. 2005 Apr;152(4):655-61. doi.org/10.1530/eje.1.01878
Luetjens CM, Stukenborg JB, Nieschlag E, Simoni M, Wistuba J. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology. 2008 Apr;149(4):1736-47. doi.org/10.1210/en.2007-1325
Ntemou E, Kadam P, Van Saen D, Wistuba J, Mitchell RT, Schlatt S, Goossens E. Complete spermatogenesis in intratesticular testis tissue xenotransplants from immature non-human primate. Hum Reprod. 2019 Mar 1;34(3):403-413. doi.org/10.1093/humrep/dey373
Pohl E, Gromoll J, Kliesch S, Wistuba J. An alternative interpretation of cellular 'selfish spermatogonial selection'-clusters in the human testis indicates the need for 3-D-analyses. Andrology. 2016 Mar;4(2):213-7. doi.org/10.1111/andr.12142
Pohl E, Höffken V, Schlatt S, Kliesch S, Gromoll J, Wistuba J. Ageing in men with normal spermatogenesis alters spermatogonial dynamics and nuclear morphology in Sertoli cells. Andrology. 2019 Nov;7(6):827-839. doi.org/10.1111/andr.12665
Wistuba J, Schlatt S. Transgenic mouse models and germ cell transplantation: two excellent tools for the analysis of genes regulating male fertility. Mol Genet Metab. 2002 Sep-Oct;77(1-2):61-7. doi.org/10.1016/s1096-7192(02)00142-7Wistuba J, Mundry M, Luetjens CM, Schlatt S. Cografting of hamster (Phodopus sungorus) and marmoset (Callithrix jacchus) testicular tissues into nude mice does not overcome blockade of early spermatogenic differentiation in primate grafts. Biol Reprod. 2004 Dec;71(6):2087-91. doi.org/10.1095/biolreprod.104.033431
Wistuba J, Marc Luetjens C, Kamischke A, Gu YQ, Schlatt S, Simoni M, Nieschlag E. Pharmacokinetics and pharmacodynamics of injectable testosterone undecanoate in castrated cynomolgus monkeys (Macaca fascicularis) are independent of different oil vehicles. J Med Primatol. 2005 Aug;34(4):178-87. doi.org/10.1111/j.1600-0684.2005.00115.x
Wistuba J, Mittag J, Luetjens CM, Cooper TG, Yeung CH, Nieschlag E, Bauer K. Male congenital hypothyroid Pax8-/- mice are infertile despite adequate treatment with thyroid hormone. J Endocrinol. 2007 Jan;192(1):99-109. doi.org/10.1677/JOE-06-0054
Wistuba J, Nieschlag E, Semjonow A, Sandhowe-Klaverkamp R, Friderichs-Gromoll S, Zitzmann M, Simoni M, Luetjens CM. Testosterone-induced prostate growth is blocked by co- and pre-administration of norethisterone enanthate (NETE) in castrated cynomolgous monkeys (Macaca fascicularis). Urologia Internationalis 2012; 88: 358-364; IF 1,065 doi.org/10.1159/000335209
Wistuba J, Luetjens CM, Ehmcke J, Redmann K, Damm OS, Steinhoff A, Sandhowe-Klaverkamp R, Nieschlag E, Simoni M, Schlatt S. Experimental endocrine manipulation by contraceptive regimen in the male marmoset (Callithrix jacchus). Reproduction. 2013 Apr 15;145(4):439-51. doi.org/10.1530/REP-12-0373
In vitro differentiation of the male germ line – maturation of male gametes in a petri dish
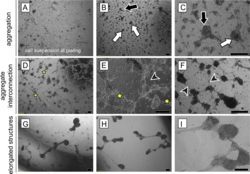
Morphological changes in the adult human testicular cell suspension in vitro. Series of representative phase-contrast micrographs revealing the sequence of typical changes of cellular reassembly. (A) The freshly prepared cell suspension consisted of single cells and small multi-cellular aggregates . (B, C) After the first 2 days of culture (B, magnified in C; designated as Day 0 of the experimental observation), cells had reassembled into irregular (black arrows) and/or round aggregates (white arrows). (D-F) By Day 1 (D magnified in E), aggregates interconnected via bundles of elongated cells (stars). In addition, individual elongated cells protruded from the aggregates (black arrowheads). (G-I) Aggregates compacted further and adjacent aggregates coalesced to form elongated structures ( H magnified in I) by Day 3. Scale bars = 200 µm. (Mincheva et al. 2018) Spermatogenesis is an extremely complex process. For over a century it has been tried to mimic maturation of germ cells in cell and organ cultures in order to understand the cooperation and communication between the somatic cells of the testis and the germ line and to achieve haploidization and sperm production from immature germ cells into spermatozoa in vitro. Despite decades of research, these approaches have remained largely experimental. In recent years, however, important principles were discovered enabling the use of new culture systems, which may be useful to differentiate mammalian sperm in vitro. Although limited in terms of efficiency, recently living mice were generated by assisted reproduction technology with sperm derived from tissue cultures for the first time by a Japanese group. In cultures with mixtures of isolated testicular mouse cells we could obtain morphologically differentiated spermatozoa after a few weeks, differentiated from diploid pre-meiotic cells placed in culture before. We used either a novel matrix-based (Soft Agar Culture System (SACS), methylcellulose Culture System (MCS), or a collagen scaffold as three-dimensional culture systems, which can also be found in regenerative medicine applications (Stukenborg et al 2009, Reuter et al. 2012, 2014). Currently we were sucessful in transferring our culture approaches to human tissues which we obtain druing the sex confirming surgery in male-to-female transgender persons (Schneider et al. 2015). We were able to show that these tissues resemble wide parts of tubulogenesis and self assembly of testicular somatic cells in vitro, offering a novel strategy for a test system for reproductive toxikology but also – more important for autologous exploration of new fertility preservation methodology (Mincheva et al 2018, 2020).
Literature:
Mincheva M, Sandhowe-Klaverkamp R, Wistuba J, Redmann K, Stukenborg JB, Kliesch S, Schlatt S. Reassembly of adult human testicular cells: can testis cord-like structures be created in vitro? Mol Hum Reprod. 2018 Feb 1;24(2):55-63. doi.org/10.1093/molehr/gax063
Mincheva M, Sandhowe-Klaverkamp R, Wistuba J, Redmann K, Stukenborg JB, Kliesch S, Schlatt S. Reassembly of adult human testicular cells: can testis cord-like structures be created in vitro? Mol Hum Reprod. 2018 Feb 1;24(2):55-63. doi.org/10.1093/molehr/gax063
Reuter K, Schlatt S, Ehmcke J, Wistuba J. Fact or fiction: In vitro spermatogenesis. Spermatogenesis. 2012 Oct 1;2(4):245-252. doi.org/10.4161/spmg.21983
Reuter K, Ehmcke J, Stukenborg JB, Simoni M, Damm OS, Redmann K, Schlatt S, Wistuba J. Reassembly of somatic cells and testicular organogenesis in vitro. Tissue Cell. 2014 Feb;46(1):86-96. doi.org/10.1016/j.tice.2013.12.001
Stukenborg JB, Wistuba J, Luetjens CM, Elhija MA, Huleihel M, Lunenfeld E, Gromoll J, Nieschlag E, Schlatt S. Coculture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J Androl. 2008 May-Jun;29(3):312-29. doi.org/10.2164/jandrol.107.002857
Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, Huleihel M, Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Hum Reprod. 2009 Sep;15(9):521-9. doi.org/10.1093/molehr/gap052
Klinefelter Syndrome as an example for the consequences of a disturbed sexchromosomal balance: the 41, XXY* mouse model
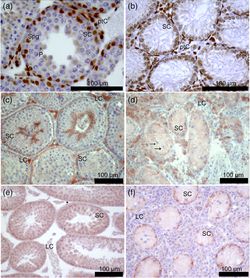
Immunohistochemical detection of the androgen receptor (AR), occludin, and ZO-1 in testicular cross sections from male mice: (a, c, e) 40, XY* control; (b, d, f) 41, XXY* Gewebe; (a, b, e, f) 21 days post partum; (c, d) adult. (Wistuba et al., 2020) Klinefelter syndrome (KS: sex-chromosomal aberration, karyotype 47, XXY, incidence of approximately 1: 600) is one of the main complex genetic disorders in men and can be seen exemplarily for effects of a disturbed sexchromosomal balance. It is caused by a non-disjunction of the sex chromosomes during meiosis in the parental generation. The condition results in a heterogeneous phenotype of variable severity. Amongst its prominent features are infertility, hypogonadism, gynecomastia, cardiovascular problems, disturbed bone metabolism, diabetes, and cognitive deficits. In all patients, the progressive loss of germ cells and hypergonadotropic hypogonadism is common. Mouse models of the strain B6Ei.LT-Y* have been successfully established at the CeRA in the last years and resemble remarkably well the human KS phenotype (Wistuba et al. 2017, 2020a). We could demonstrate that body proportions of our animal model for KS, 41,XXY* male mice, are altered, that they develop hypergonadotropic hypogonadism in adulthood, and are infertile due to progressive germ cell loss. We also found these male mice to exhibit disturbed memory recognition. Leydig cell function or maturation had been suggested for years and by several groups to be disturbed resulting in insufficient response to endocrine stimulation due to LH receptor dysfunction or steroidogenic failure. Alternatively, Leydig cell hyperplasia was discussed as a putative consequence of incomplete maturation. However, we recently questioned these concepts by demonstrating in our mouse model Leydig cells to be mature and (hyper)active (Wistuba et al. 2010) and that inmice and KS patients intratesticular T (ITT) values were not different from controls but at least the mice exhibited altered testicular blood flow as shown by fucntional experiments using contrats enhanced ultrasound (Tüttelmann et al. 2014, Wistuba et al. 2020b). These exciting results put forward a new concept on the origin of hypergonadotropic hypogonadism but also demonstrate the validity of our mouse model, with its translational consequences for our understanding of KS patients. This was convincingly shown in stuides analysing similar questions in a patient cohort (Zitzmann et al 2015, Rohayem et al 2016); an we were even able to contribute tot he detection of a sofar unknown ocular phenotyope in KS men (Brand et al. 2017). The availability of our mouse model, the option to analyze gene expression patterns and somatic cell responses and the close cooperation between clinics and basic science in our Centre puts us in the privileged situation not only to study aspects of the mechanisms by which e.g. escapee genes provoke a complex phenotype but also to directly translate those findings into a clinical context (Wistuba et al. 2020a).
Our work with the KSmouse model awarded several grants by the faculty and the DFG and we were able to win the Dietrich-Knorr Price of the German Society for Endocrinology fort he best pubication in reproductive endocrinology twice (Wistuba et al 2010, Werler et al. 2014).Literature:
Brand C, Zitzmann M, Eter N, Kliesch S, Wistuba J, Alnawaiseh M, Heiduschka P. Aberrant ocular architecture and function in patients with Klinefelter syndrome. Sci Rep. 2017 Oct 13;7(1):13130. doi.org/10.1038/s41598-017-13528-4Tuttelmann F, Damm OS, Luetjens CM, Baldi M, Zitzmann M, Kliesch S, Nieschlag E, Gromoll J, Wistuba J, Simoni M. Intratesticular testosterone is increased in men with Klinefelter syndrome and may not be released into the bloodstream owing to altered testicular vascularization– a preliminary report. Andrology. 2014 Mar;2(2):275-81. doi.org/10.1111/j.2047-2927.2014.00190.x
Werler S, Demond H, Damm OS, Ehmcke J, Middendorff R, Gromoll J, Wistuba J. Germ cell loss is associated with fading Lin28a expression in a mouse model for Klinefelter's syndrome. Reproduction. 2014 Jan 25;147(3):253-64. doi.org/10.1530/REP-13-0608
Wistuba J. Animal models for Klinefelter's syndrome and their relevance for the clinic. Mol Hum Reprod. 2010 Jun;16(6):375-85. doi.org/10.1093/molehr/gaq024
Wistuba J, Werler S, Lewejohann L. Mouse models for the exploration of Klinefelter´s syndrome. In Conn MJ (ed) Animal models of human diseases, Elsevier, 2013, 759-784, doi.org/10.1016/B978-0-12-809468-6.00024-3
Wistuba J, Werler S, Lewejohann L, Brand C, Damm OS. Mouse models for the exploration of Klinefelter´s syndrome. In Conn MJ (ed) Animal models for the study of human diseases; 2nd revised edition, Elsevier Academic Press, ISBN 9780128094686, published date: 8th June 2017, Page Count: 1182 , 2017, pages 617-645
Wistuba J, Beumer C, Brehm R, Gromoll J. 41,XXY * male mice: An animal model for Klinefelter syndrome. Am J Med Genet C Semin Med Genet. 2020 Jun;184(2):267-278. doi.org/10.1002/ajmg.c.31796
Wistuba J, Beumer C, Warmeling AS, Sandhowe-Klaverkamp R, Stypmann J, Kuhlmann M, Holtmeier R, Damm OS, Tüttelmann F, Gromoll J. Testicular blood supply is altered in the 41,XXY* Klinefelter syndrome mouse model. Sci Rep. 2020 Sep 1;10(1):14369. doi.org/10.1038/s41598-020-71377-0Zitzmann M, Bongers R, Werler S, Bogdanova N, Wistuba J, Kliesch S, Gromoll J, Tüttelmann F. Gene expression patterns in relation to the clinical phenotype in Klinefelter syndrome. J Clin Endocrinol Metab. 2015 Mar;100(3):E518-23. doi.org/10.1210/jc.2014-2780

