






How do sperm find their way to the egg? Our primary research focus is to answer this fundamental question in reproductive research.
To this end, we investigate the complex interplay of receptors, ion channels, and chemical messengers that enable sperm to translate changes in their chemical and physical microenvironment into a behavioral response. From electrophysiology to fluorescent probes, high-speed motility studies, and protein biochemistry – we rely on a broad range of state-of-the-art methods to tackle this demanding task. For us, however, sperm are more than just a powerful model organism for basic research in cellular signal transduction: At the limits of our present knowledge about the molecular basics of human reproduction, we are looking for the answers behind unexplained male infertility.
We want to decipher the signaling molecules and pathways employed by sperm to translate changes in the physicochemical microenvironment into a behavioral response. In particular, we investigate, using a battery of cell biological, protein biochemical, and fluorometric techniques, the basic principles of sperm chemosensation. Our work concerning the action of chemical cues released into the semen and oviduct as well as of environmental chemicals on human sperm has already provided several important insights.
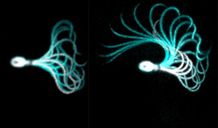
How do physicochemical signals translate into changes of sperm motility? What is it that guides sperm towards the egg and allows them to break through its rigid protective coats? A unique set of advanced microscopic techniques, ranging from state-of-the-art high-speed microscopic video acquisition to self-designed optical tweezers, microfluidics, and microhydraulic setups for kinetic stopped-flow microscopy allows us to study the swimming behavior of sperm. Our group combines scientific skills and boundless creativity to study sperm chemotaxis and rheotaxis, the navigation along chemical cues and fluid flows, respectively.

Selected literature:
Schiffer, C., Rieger, S., Brenker, C., Young, S., Hamzeh, H., Wachten, D., Tuttelmann, F., Ropke, A., Kaupp, U. B., Wang, T., Wagner, A., Krallmann, C., Kliesch, S., Fallnich, C. & Strunker, T. (2020) "Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca(2+) signaling" The EMBO journal 39, e102363. doi.org/10.15252/embj.2019102363
Brenker, C., Schiffer, C., Wagner, I. V., Tuttelmann, F., Ropke, A., Rennhack, A., Kaupp, U. B. & Strunker, T. (2018) "Action of steroids and plant triterpenoids on CatSper Ca(2+) channels in human sperm" Proceedings of the National Academy of Sciences of the United States of America 115, E344-E346. doi.org/10.1038/emboj.2012.30
Schiffer, C., Muller, A., Egeberg, D. L., Alvarez, L., Brenker, C., Rehfeld, A., Frederiksen, H., Waschle, B., Kaupp, U. B., Balbach, M., Wachten, D., Skakkebaek, N. E., Almstrup, K. & Strunker, T. (2014) "Direct action of endocrine disrupting chemicals on human sperm" EMBO reports. doi.org/10.15252/embr.201438869
Brenker, C., Goodwin, N., Weyand, I., Kashikar, N. D., Naruse, M., Krahling, M., Müller, A., Kaupp, U. B. & Strünker, T. (2012) "The CatSper channel: a polymodal chemosensor in human sperm" EMBO J. 31, 1654-1665. doi.org/10.1038/emboj.2012.30
Strünker, T., Goodwin, N., Brenker, C., Kashikar, N. D., Weyand, I., Seifert, R. & Kaupp, U. B. (2011) "The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm" Nature 471, 382-386. doi.org/10.1038/nature09769

We study the molecular and cellular pharmacology, physiology, and biophysics of ion channels and transporters controlling sperm function and fertilization. CatSper, a promiscuous polymodal sensor for a plethora of physicochemical stimuli, takes center stage in the regulation of sperm behavior – and in our research. CatSper is embedded into a complex network of membrane proteins governing sperm signal transduction: The potassium channel Slo3 and the proton channel Hv1, key players in the regulation of membrane potential and intracellular pH, respectively, are also fascinating subjects of our scientific endeavors. With the development of novel inhibitors for sperm ion channels, we provide tools for basic research in reproduction and blueprints for novel contraceptives.
Selected literature:
Wang, T., Young, S., Krenz, H., Tüttelmann, F., Röpke, A., Krallmann, C., Kliesch, S., Zeng, X., Brenker, C., & Strünker, T. (2020) "The Ca2+ channel CatSper is not activated by cAMP/PKA signaling but directly affected by chemicals used to probe the action of cAMP and PKA." Journal of Biological Chemistry. doi.org/10.1074/jbc.RA120.013218
Brenker, C., Zhou, Y., Muller, A., Echeverry, F.A., Trotschel, C., Poetsch, A., Xia, X.M., Bönigk, W., Lingle, C.J., Kaupp, U.B. & Strünker, T. (2014) "The Ca2+-activated K+ current of human sperm is mediated by Slo3" eLife 3. doi.org/10.7554/eLife.01438
Rennhack A, Schiffer C, Brenker C, Fridman D, Nitao ET, Cheng YM, Tamburrino L, Balbach M, Stölting G, Berger TK, Kierzek M, Alvarez L, Wachten D, Zeng XH, Baldi E, Publicover S, Kaupp UB, Strünker T (2018). "A novel cross-species inhibitor to study the function of CatSper Ca2+ channels in sperm." Br J Pharmacol. doi.org/10.1111/bph.14355
Our research aims to develop new diagnostics for the early detection of male infertility, as well as evidence-based therapy selection in fertility medicine.
As part of a project funded by EFRE.NRW and the EXIST Transfer of Research programme, a project team led by Dr Christian Schiffer and Dr Samuel Young — former members of our working group who are now guest scientists — has converted a laboratory test, the CatSper test, into a CE-compliant medical device. The CatSper test can be used to detect CatSper-related infertility.
You can find out more about the CatSper test on the website of our spin-off company, Truion.
Selected Literature:
Young, S.*, Schiffer, C.*, Wagner, A., Patz, J., Potapenko, A., Herrmann, L., Nordhoff, V., Pock, T., Krallmann, C., Stallmeyer, B., Röpke, A., Kierzek, M., Biagioni, C., Wang, T., Haalck, L., Deuster, D., Hansen, J. N., Wachten, D., Risse, B., Behre, H. M., Schlatt, S., Kliesch, S., Tüttelmann, F.*, Brenker, C.*, Strünker, T.* (2024) “Human fertilization in vivo and in vitro requires the CatSper channel to initiate sperm hyperactivation” Journal of Clinical Investigation 134(1):e173564 doi.org/10.1172/JCI173564
Schiffer, C., Rieger, S., Brenker, C., Young, S., Hamzeh, H., Wachten, D., Tuttelmann, F., Ropke, A., Kaupp, U. B., Wang, T., Wagner, A., Krallmann, C., Kliesch, S., Fallnich, C. & Strunker, T. (2020) "Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca(2+) signaling" The EMBO journal 39, e102363. doi.org/10.15252/embj.2019102363