These pages are currently under construction.
Research Topics
Idiopathische männliche Infertilität
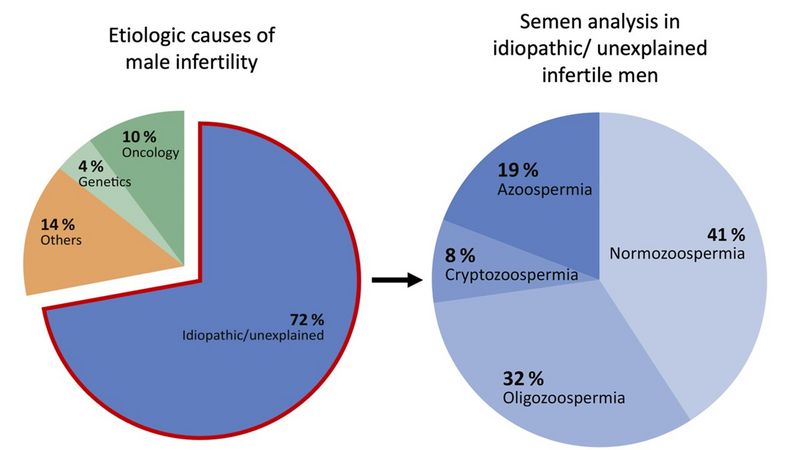
adapted from Tüttelmann et al., 2018 In Western countries, at least 15% of couples of reproductive age are affected by infertility, with a male factor contributing significantly in 50% of cases. Non-genetic causes—such as high-grade varicocele, a history of undescended testis (maldescensus testis), or prior treatment for oncological diseases—can explain impaired fertility in approximately one in four patients (see figure).
However, in the majority of infertile men, genetic factors are believed to play a key role. In these cases, infertility can generally be categorized into a rare, classical monogenic form (approximately 4% of cases) and a much more common (>70%) multifactorial form, also referred to as genetically complex. The latter involves a combination of common genetic factors, environmental influences, and lifestyle factors.
The cause of infertility in these men is currently unknown and is classified as:
i) idiopathic, when semen parameters are impaired (e.g., low sperm count – oligozoospermia), and
ii) unexplained, when semen analysis reveals normal sperm parameters (normozoospermia) (Schlegel et al., 2021). Despite the heterogeneous phenotype of total sperm count (TSC) in these men, they share the common clinical feature of male infertility. The goal of the Translational Andrology research group is to reduce the number of idiopathically infertile men by identifying additional etiological factors.Through various projects, we have already made progress in this area, with a clear focus on analyzing the relevance of genotyping the FSHB polymorphism (FSHB c.-211 G>T) in male infertility. In our current projects, we have further investigated the significance of this polymorphism using techniques such as genome-wide association studies (GWAS), histopathological analysis of testicular tissue samples, and clustering of a large patient cohort (a form of machine learning/artificial intelligence).
These approaches have allowed us to demonstrate the impact of the FSHB c.-211 G>T polymorphism on FSH serum levels, Sertoli cells (supporting cells) in the testis, and its clinical relevance in the context of fertility diagnostics.
Further Literature
Schwarzkopf V, Wistuba J, Sandhowe-Klaverkamp R, Kliesch S, Gromoll J, Schubert M. Unraveling a subgroup of men with unexplained male infertility - men with normogonadotropic non-obstructive azoospermia. J Clin Endocrinol Metab. 2025 Apr. doi: 10.1210/clinem/dgaf200.
Silva AFN*, Schubert M*, Kliesch S, Schlatt S, Ramalho-Santos J, Amaral SCG. Prevalence and impact of antisperm antibodies on semen quality and male reproductive health aspects: A 10-years retrospective study. Andrology. 2025 Mar 11. doi: 10.1111/andr.70020.
Schubert M*, Pérez Lanuza L*, Wöste M, Dugas M, Carmona FD, Palomino-Morales RJ, Rassam Y, Heilmann-Heimbach S, Tüttelmann F, Kliesch S, Gromoll J. A GWAS in Idiopathic/Unexplained Infertile Men Detects a Genomic Region Determining Follicle-Stimulating Hormone Levels. J Clin Endocrinol Metab. 2022 Jul. doi: 10.1210/clinem/dgac165.
Krenz H, Sansone A, Kliesch S, Gromoll J, Schubert M. FSHB Genotype Identified as a Relevant Diagnostic Parameter Revealed by Cluster Analysis of Men With Idiopathic Infertility. Front Endocrinol (Lausanne). 2021 Dec. doi: 10.3389/fendo.2021.780403.
Schubert M, Kaldewey S, Pérez Lanuza L, Krenz H, Dugas M, Berres S, Kliesch S, Wistuba J, Gromoll J. Does the FSHB c.-211G>T polymorphism impact Sertoli cell number and the spermatogenic potential in infertile patients? Andrology. 2020 Sep. doi: 10.1111/andr.12777.
Infertility and Comorbidities
Most men with infertility are otherwise healthy at the time of fertility workup. However, several studies have repeatedly shown that infertile men are at increased risk for developing comorbidities, while the causal links between these conditions remain mostly unclear. Associations between male infertility and comorbidities were specifically described for cardiovascular disease and consecutive death, high blood pressure, diabetes mellitus type 2, metabolic syndrome, autoimmune diseases (like Multiple Sclerosis), testicular germ cell cancer and prostate cancer. Many of these studies are based on health records or observational studies, with a diagnosis of ‘infertility’ based on questionnaire data or the review of medical records. As a consequence, they often lack deep phenotypic characterization of patients and do not consider the underlying heterogeneity (in causes) of infertility.
For some infertile conditions, like ‘secondary hypogonadism’ for instance, where reduced testosterone may serve as risk factor for future comorbidity the causal relation is obvious.
However, in the majority of infertile men causative factors leading to impaired fertility can not be identified, yet. Male infertility of yet unknown etiology, i.e. ‘idiopathic’ (semen parameters affected) or ‘unexplained’ (semen parameters unaffected) infertility is likely caused by a combination of common genetic, environmental, and lifestyle factors. However, the interplay of these factors is currently understudied, therewith diagnostic accuracy is reduced, which leads to a large fraction of pathomechanistically unexplained infertility, and precludes personalised medicine and the identification of a putative shared etiologic basis of comorbidities and infertility.
Our research group is approaching this topic through two projects:
A) In collaboration with Prof. Dr. André Karch (see Collaborations and Grants), Prof. Dr. Sabine Kliesch, Mattia Anfosso, and Dr. Simone Bier, we are conducting a re-evaluation of infertile men, focusing on changes in andrological parameters over time and on potentially newly developed comorbidities. As part of this project, former patients are being recontacted and invited for follow-up visits. In the future, the collected data will be compared with the epidemiological data from the NAKO (German National Cohort).
B)Male infertility of yet unknown etiology, i.e., ‘idiopathic’ or ‘unexplained’ infertility is likely caused by a combination of common genetic, environmental, and lifestyle factors. In this context, genome-wide association studies (GWAS) have the potential to significantly contribute to identifying novel genetic risk factors for male infertility and related traits. For instance, we have previously shown that single nucleotide polymorphisms (SNPs) alter Follicle-stimulating hormone (FSH) levels and thereby contribute to the underlying etiology.
In a cooperation, together with Prof. Dr. Christina Lill, and Dr. Olena Ohlei, we aim to identify new candidate SNPs together with the prior described FSH-associated SNPs, which will yield a panel of genetic risk factors for idiopathic and unexplained male infertility. This may pave the way to improved phenotyping and therewith carry forward diagnostics in male infertility workup.
Knowledge, experience, and decision-making in the context of andrological diagnostics and therapeutic (recommendations) in male infertility
The diagnostic and therapeutic process in cases of involuntary childlessness is a particular challenge for couples. There has been little research on the experiences of men in particular - especially when the reason for the lack of pregnancy may lie on the male side. We would like to change this with our study.
In a cooperation, together with Dr. Markus Kluge, we are specifically interested in how men experience the diagnosis and initial treatment process for involuntary childlessness, what challenges they face and what decisions they make for themselves (and together with their partner). In particular, we want to understand the importance of non-clinical media, sources of information and knowledge.
Three interviews of approx. 45 minutes each will be conducted as part of the study.

