Establishing a Controlled Human Hookworm Infection Model in Lambaréné, Gabon: a platform to foster the development of intervention against soil-transmitted helminths.
Establishing a controlled Human Hookworm Infection (CHHI) Model in a sub-Saharan African setting where STH infections are highly endemic is unprecedented. The CHHI in Africa (CHHIA) is essential to fulfil several unmet scientific objectives.
Genomic and post-genomic characterisation of local strains of Hookworm
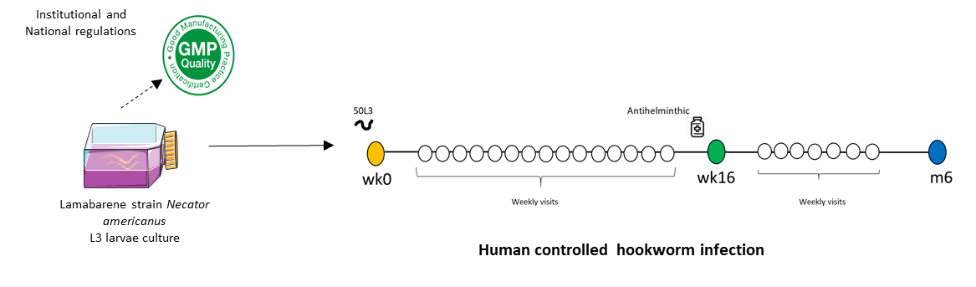
Production of stage 3 Necator americanus larvae from naturally infected donors living in Lambaréné ( L3 Lambaréné Na larvae), that will meet the criteria of an investigational product: clean, safe, Good Manufactory Practice( GMP) compliant and medical grade product ( batch specifications are phenotypic classification, potency of infectivity, parasitism and immune escape)
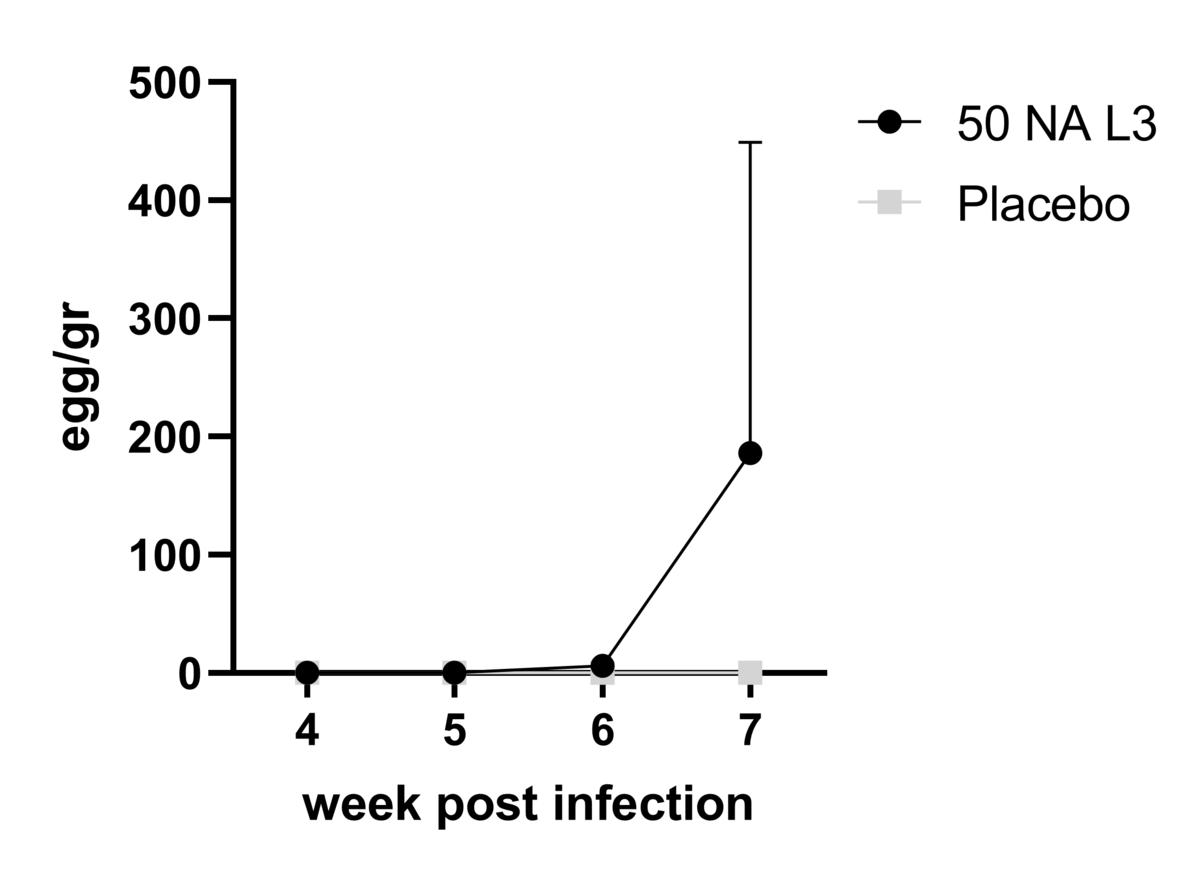
Identification of the infective dose of L3 Lambaréné Na and safety profile of CHHIA
Cryopreservation of L1-L3-produced larvae
Screening of new or improved vaccine targets using antibodies to protein arrays of Necator americanus L1-L3 larvae
Investigation of vaccine adjuvant candidates through cellular immune responses, especially those with prominence in the activation of the innate response
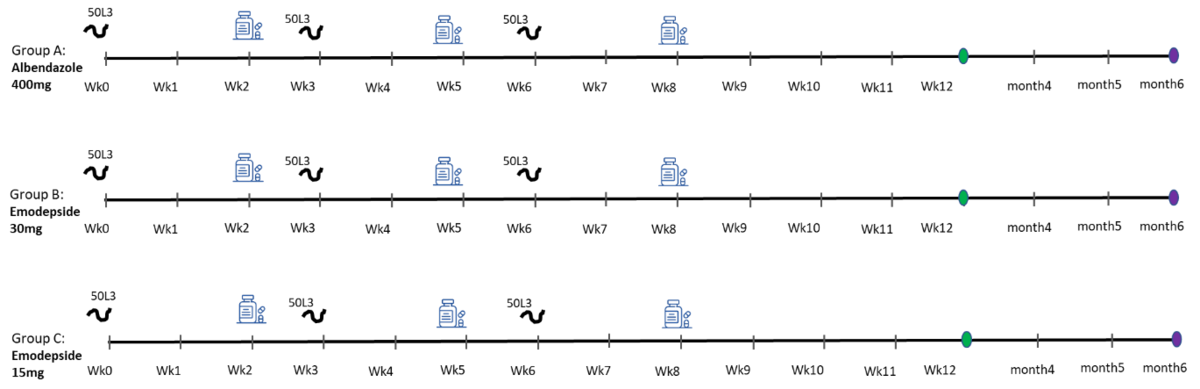
Screening of antihelminth resistance-breaking compounds
Accelerating clinical evaluation of promising antihelminth vaccine and drug candidates