Research foci
DNA methylation and male fertility
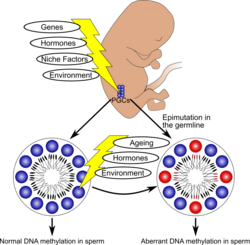
Model for the occurrence of epigenetic aberrations in human sperm (adapted from Laurentino et al. 2016). Epigenetics is the study of inheritable changes to gene expression that are not associated with changes to gene sequence. Epigenetic marks include histone modifications, non-coding RNAs, and DNA/cytosine methylation. Imprinted genes are diallelic genes which are expressed in a mono-allelic, parent-of-origin-dependent manner. Paternally imprinted genes (e.g. H19) are usually hypermethylated (silenced) in the sperm and hypomethylated in the oocyte, and therefore in the offspring they are expressed through the maternally inherited copy. With maternally imprinted genes (e.g. MEST) it is the other way around. In recent years, defective sperm DNA methylation in several genes (including imprinted genes) has been associated with male infertility. Moreover, factors like ageing might influence sperm DNA methylation in regions not involved with genetic imprinting. In our group, we are interested in understanding changes to sperm DNA methylation.
Ageing in male germ cells
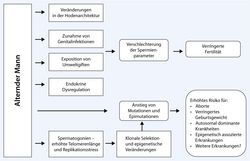
The effects of age on male fertility and the descendants (Gromoll et al. 2016). The tendency for delayed parenthood is a widespread phenomenon in societies of industrial countries. Effects resulting from advanced female age are a decline in fecundity and an increased risk for chromosomal abnormalities, e.g. trisomy 21 causing Down syndrome. However, the impact of advanced paternal age on fertility and health for the offspring has not been sufficiently investigated. There is evidence that ageing is associated with a decline in sperm parameters and an increased risk for some congenital disorders in the offspring. Even though the exact mechanisms behind the paternal age effect (PAE)-disorders are not fully understood yet. Several studies point towards an age-dependent increase in de novo mutation-rate in male germ cells and an increase in DNA-fragmentation in sperm from older men. A further age-dependent change in sperm is the significant increase in telomere length.
Besides the genetic factors it was proposed that also epigenetic characteristics, such as aberrant DNA methylation, are related to infertility and health impairments in the offspring. For instance, age-dependent changes in DNA methylation signatures in sperm were found in genes associated with schizophrenia and bipolar disorders. Moreover, aberrant DNA methylation patterns are significantly elevated in sperm from infertile man.
The aim of our study is to define age-dependent changes in male germ cells in a group of healthy and clinically characterised donors at a genetic and epigenetic level. As a long-term goal these baseline values will later on serve as a reference for comparative analyses in infertility patients. For this we started a clinical study in which more than 200 healthy probands (age 20-83 years) were recruited and sperm and blood samples collected. We now have started to characterise the integrity of their spermatozoa during lifespan to estimate the possible risks of older fathers.Selected literature:
Laurentino, S., Cremers, J.-F., Horsthemke, B., Tüttelmann, F., Czeloth, K., Zitzmann, M., … Gromoll, J. (2019). Healthy ageing men have normal reproductive function but display germline-specific molecular changes. MedRxiv, 19006221. https://doi.org/10.1101/19006221
© UM, S. Berres, graphic: W. Kramer Functional and clinical role of genetic polymorphisms in the FSHB and FSHR genes
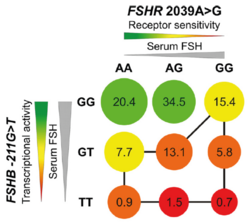
Proposed model of FSHB −211G>T and FSHR 2039A>G effects: Decreasing transcriptional activity (FSHB −211G>T, stronger effect) and receptor sensitivity (FSHR 2039A>G, weaker effect) both lead to a decrease of testicular volume depicted by circle diameter. Colors represent genotypes with better (green) and worse (red) putative impact on reproductive fitness. Numbers in circles reflect percentage of carriers of combined genotypes in the studied population. The lowest testicular volumes are predicted for TT/GG carriers. Those men with the least favorable genotype combinations are connected with the black line. 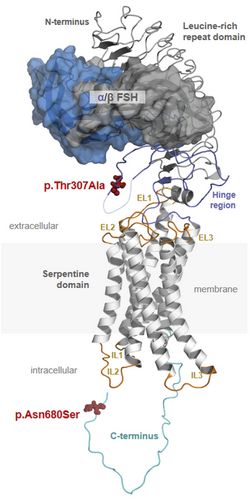
Protein structure of FSH and FSHR. The complex consists of FSH (upper part) and FSHR (lower part). FSH contains subunits α and β and FSHR consists of extracellular, membrane and intracellular domains. (3-D model by Gunnar Kleinau, Charité Berlin) The pituitary hormone FSH is essential for the initiation and maintenance of spermatogenesis in humans. Recently we could show that single nucleotide polymorphisms (SNPs) in the FSHB or the FSHR could significantly change FSH action and thereby spermatogenesis. We developed a model (Fig.3) which depicts the effect of favorable and non-favorable SNP combinations on testicular volume and sperm count. Currently we are addressing the molecular mechanisms underlying these SNPs in experimental and clinical studies.
Although the so far identified SNPs are candidates for clinical decisions on FSH treatment, they only explain a small percentage of FSH serum variation, highlighting the need for further broadening the genetic stratification. We hypothesize that deciphering the FSH signalling network and its interacting genetic variants will identify a subgroup of infertile patients in which spermatogenesis could be stimulated by FSH. Therefore, we will i) conduct GWAS to identify novel genetic variants regulating the FSH network in a highly defined and fully characterized cohort of patients with an amplified effect size followed by a validation study ii) explore the FSH secretagogue and FSHR signalling pathways, and iii) perform functional studies on the identified genetic variants. This interaction of clinical, genetic and experimental research will lead to a defined subset of functional SNPs affecting FSH action on spermatogenesis in infertile men (FSH SNP-panel). The FSH network will decipher the intricate interaction between germ and Sertoli cells. Thus, our study will give important insights into FSH action on spermatogenesis and identify novel endocrine and genetic factors causing male infertility, thereby fuelling the ‘Male Fertility Gene Atlas’. In perspective, this project could provide a treatment regimen for male infertility with improved pregnancy rates and reduced risks for the offspring as well as the psychological and socioeconomic burden of ART treatment.
Characterization of genotype and phenotype of males with 46,XX testicular Disorders of Sex Development (TDSD)
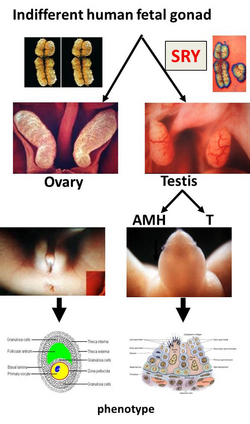
The process of sexual development. The first step of sexual determination is genetically controlled, whereas the further differentiation is controlled through hormones. After that, the sex will be maintained. 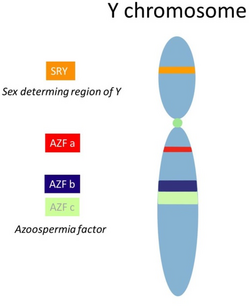
Y chromosome with the SRY gene on the short arm and the AZF region on the long arm. The SRY gene plays a major role in the testis development while the AZF region contains different genes essential for a regular spermatogenesis. 46, XX TDSD is a rare form of Disorders of Sex Development, characterized by a female genotype, however a male phenotype. In most cases the SRY gene is translocated onto a different chromosome, allowing testis development. In the case of SRY negative 46, XX TDSD other genes involved in the sexual differentiation are mutated. Due to the lack of other Y chromosomal material including the AZF region patients suffer from Azoospermia.
Despite previous studies, much of the genetic, epigenetic and clinical background of 46, XX TDSD is still unclear to date. As our institute has access to the world’s largest cohort of 46, XX TDSD, we are interested in examining the effect of the genetic characteristics on the physical, genetic, epigenetic and neurocognitive status. Some of these projects will be performed in collaboration with the Institute of Human Genetics and the Clinic for Psychiatry and Psychotherapy.

