Resistance-appropriate Helicobacter pylori eradication and antibiotic stewardship
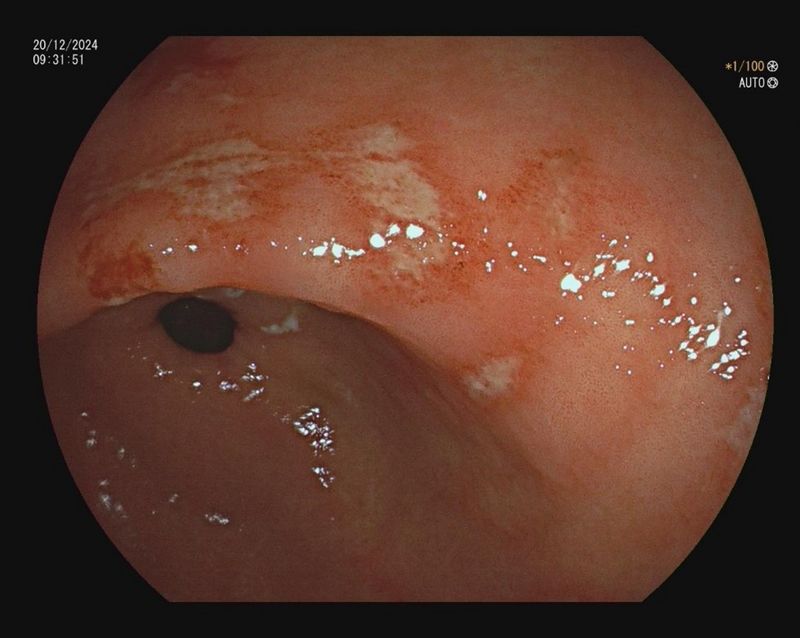
Endoskopische Ulcera sind u.a. eine Indikation für eine Helicobacter pylori Diagnostik. Im positiven Fall streben wir im Rahmen der Studie im Gegensatz zur Leitlinie keine empirische Therapie, sondern eine resistenzgerechte Therapie an.
An infection with H. pylori (HP) is often diagnosed as the cause of abdominal diseases. The resulting eradication therapy is usually carried out according to the DGVS 2022 guideline (LL) using the ‘quadruple regimen’ (Pylera®), although this is based on metronidazole, among other things, to which high resistance rates are known in some regions. The LL only provides for resistance testing - which is possible in principle - after one or two treatment failures.
We want to question this procedure and instead propose a more comprehensive resistance determination for resistance-appropriate antibiotic therapy.
The aim of the study is comprehensive resistance testing of samples with an HP-positive urease test for the initiation of resistance-appropriate therapy. The idea is that this approach will prevent treatment failure and the associated need for repeat diagnostic sampling. In addition, we can collect regional resistance data, save antibiotics and positively influence the development of resistance in the sense of antibiotic stewardship. Further information can be found in the German Register for Clinical Trials: https://drks.de/search/de/trial/DRKS00033330/details