Research focus
Biology of testicular stem cells
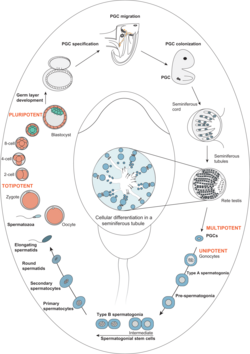
Developmental pathway illustrating male germline stem cell development and maturation from primordial germ cells to spermatozoa (Sharma et al., 2019, Human Reproduction Update). Spermatogonia are the diploid cells locates at the basemenmt membrane of the seminiferous epithelium. They arise from pluripotent precursors which develop during gonadal sexual differentiation into stem cells. These are the reserve cells which after puberty give rise to differentiating germ cells by mitotic divisions before they enter meiosis. The Schlatt team explores all aspects of spermatogonial biology. The main focus is currently put on exploration of basic mechanisms controlling formation and regulation of spermatogonial stem cells.
Research themes:
- Species specific differences in the regulation of testicular stem cells
- Interaction of spermatogonia with the somatic environment (Stem cell niche)
- Spermatogonial markers and characterisation of subpopulations
- The role of hormones and growth factors during initiation of spermatogenesis
- Mechanisms of selection and identification of check points in spermatogonia
Selected publications:
Sharma S, Wistuba J, Pock T, Schlatt S, Neuhaus N. Spermatogonial stem cells: updates from specification to clinical relevance. Hum Reprod Update. 2019;25:275-297. doi: 10.1093/humupd/dmz006
Sharma S, Portela JMD, Langenstroth-Röwer D, Wistuba J, Neuhaus N, Schlatt S. Male germline stem cells in non-human primates. Primate Biol. 2017; 4:173-184. doi: 10.5194/pb-4-173-2017
Schlatt S, Ehmcke J. Regulation of spermatogenesis: an evolutionary biologist's perspective. Semin Cell Dev Biol. 2014, 29:2-16. doi: 10.1016/j.semcdb.2014.03.007
Ex vivo Spermatogenesis
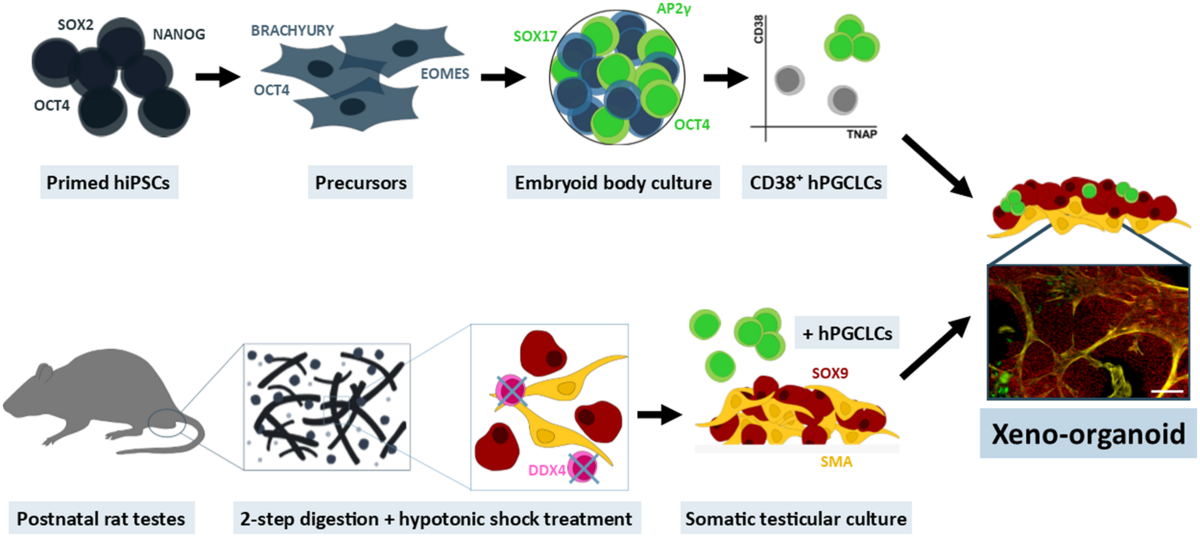
Xeno-organoid approach to explore male germ cell differentiation from hiPS-derived germ cells (Mall et al., 2020, Mol Hum Reprod). 
Microfluidic platform for the ex vivo culture of primate testis tissue (Sharma et al., 2020, Mol Hum Reprod). 
Seminiferous cord-like structures established from single cell suspensions of adult human testes obtained during sex-reassignment surgery of trans-women (Mincheva et al., 2020, Sci Rep.). 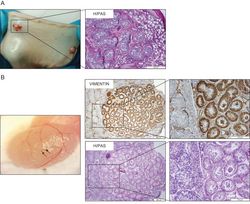
Recovery of ectopic marmoset testis xenotransplants in the back skin of immunodeficient mice (A). The grafts contain seminiferous tubules with germ cells (B). (Ntemou et al., 2019, Hum Reprod) In vitro systems to generate sperm are in the focus of scientists for more than hundred years. As yet, no efficient in vitro approaches were described to induce or maintain the full process of spermatogenesis starting from stem cells to reach mature spermatozoa. Such system are desirable to develop experimental model systems as well as for generation of gametes in the petri dish. They are considered highly relevant for veterinary and human applications. The research team runs a long term research program to explore and develop systems for maturation of testicular tissue and induction of spermatogenesis. In this context a range of animal models and diverse in vitro systems are in use. Currently, methods of grafting or transplanting germ cells and testis tissues as well as construction of testicular organoids are developed and optimised.
Research themes:
- Culture and transplantation of spermatogonia
- Xenografting of primate testes for fertility preservation
- Establishment of 3D-culture und organoid models
- In vitro recapitulation of testicular organogenesis
- Optimizing microfluidic systems for induction and maintenance of spermatogenesis ex vivo
- Characterisation of germ cell development in testes of Trans*Females
Selected publications:
Sharma S, Venzac B, Burgers T, Le Gac S, Schlatt S. Microfluidics in male reproduction: is ex vivo culture of primate testis tissue a future strategy for ART or toxicology research? Mol Hum Reprod. 2020; 26:179-192. doi: 10.1093/molehr/gaaa006
Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, Huleihel M, Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Hum Reprod. 2009;15:521-9. doi: 10.1093/molehr/gap052
Florian Schneider, Bettina Scheffer, Jennifer Dabel, Laura Heckmann, Stefan Schlatt, Sabine Kliesch, Nina Neuhaus. Options for Fertility Treatments for Trans Women in Germany. J Clin Med. 2019; 8: 730. doi:10.3390/jcm8050730
Optical methods for sperm and germ cell diagnostics
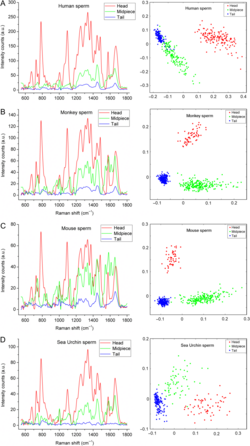
Raman spectra (left panels) and principal component analysis (right panels) for different regions of spermatozoa from different species. Man (A), Monkey (B), Mouse (C) and Sea Urchin (D). (Amaral et al., 2018, Mol Hum Reprod) Spermatozoa and germ cells can be explored using novel optical methods. This may enable their deeper characterization and selection. Multispectral analysis by flow cytometry provides tools for functional separation of cell fractions. Raman-spectroscopy allows description of biochemical components in different spermatozoal compartments. Both strategies are currently tested on human sperm by Dr. Raul Da Costa. It is the primary goal to obtain procedures leading to new diagnostic tools for sperm analysis and selection.
Research themes:
- Raman micro-spectroscopy for sperm analysis
- Multispectral flow cytometry for classification of human sperm
Selected publications:
Amaral S, Da Costa R, Wübbeling F, Redmann K, Schlatt S. Raman micro-spectroscopy analysis of different sperm regions: a species comparison. Mol Hum Reprod. 2018;24:185-202. doi: 10.1093/molehr/gax071.
Da Costa R, Amaral S, Redmann K, Kliesch S, Schlatt S. Spectral features of nuclear DNA in human sperm assessed by Raman Microspectroscopy: Effects of UV-irradiation and hydration. PLoS One. 2018; 13:e0207786. doi: 10.1371/journal.pone.0207786.
Sánchez V, Redmann K, Wistuba J, Wübbeling F, Burger M, Oldenhof H, Wolkers WF, Kliesch S, Schlatt S, Mallidis C. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil Steril. 2012;98(5):1124-9.e1-3. doi: 10.1016/j.fertnstert.2012.07.1059.
Mallidis C, Sanchez V, Wistuba J, Wuebbeling F, Burger M, Fallnich C, Schlatt S. Raman microspectroscopy: shining a new light on reproductive medicine. Hum Reprod Update. 2014;20:403-14. doi: 10.1093/humupd/dmt055.

