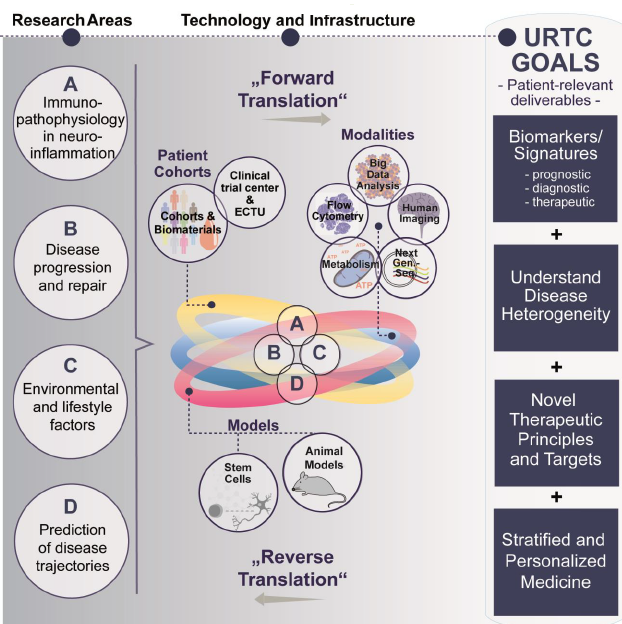
Incorporating Detailed Analysis of Immune Processes in (Neuro)Inflammatory Diseases
Multidimensional analysis of immune cells in multiple sclerosis (MS) using flow cytometry has revealed four distinct immunological endophenotypes — stable, genetically influenced neurobiological disease correlates. Notably, two of these immune signatures — the inflammatory and degenerative types — are associated with specific clinical features and respond differently to immunotherapies. Building on these groundbreaking findings, the URTC aims to identify disease-specific endophenotypes across a range of inflammatory diseases. Additionally, predictive scores for disease progression will be developed and validated using data from the pilot phase, ongoing projects, and existing cohorts.
This approach is designed to (1) provide comprehensive insights into the specific immunobiology underlying various neurological and neuropsychiatric disorders. These include neuroinflammatory diseases such as neuromyelitis optica, Susac syndrome, autoimmune encephalitis, and myasthenia gravis; organ-specific autoimmune diseases like rheumatoid arthritis and psoriasis; and psychiatric conditions known to involve peripheral immune signature changes, such as affective disorders, (2) investigate whether specific immune signatures during therapy can help predict therapeutic success, (3) adapt the research approach to facilitate integration into routine clinical practice.
Role of Inflammatory Mechanisms in Progression and Regeneration
When do repair mechanisms succeed, and when do they fail? To better understand the determinants of progression and regeneration in chronic inflammatory diseases, we are characterizing both successful and unsuccessful repair mechanisms across various tissues and organs. This translational approach enables us to identify organ-specific as well as cross-organ mechanisms that promote tissue repair.
Environmental and Lifestyle Factors vs. Genetics in Autoimmune Pathology
Autoimmune diseases are believed to arise from a complex interplay between genetic predispositions and environmental influences. Twin studies are particularly useful in disentangling the respective contributions of these factors. During the pilot phase, we identified a population of inflammatory CD4+ T cells present in prodromal MS. In the current consolidation phase, we aim to investigate: (1) how environmental factors influence the onset and progression of autoimmunity in the central nervous system (CNS). (2) how these factors can be targeted through therapeutic interventions. Two environmental factors — conjugated linoleic acid (CLA) and UV light — have demonstrated significant effects on the immune system. The URTC is currently preparing a phase II clinical trial to evaluate the impact of CLA and probiotics as adjuncts to immunotherapy in patients with relapsing-remitting MS. Additionally, immune cell metabolism has become a topic of growing scientific interest. Differences in the metabolism of healthy versus diseased immune cells offer new insights into autoimmune mechanisms. URTC will now explore this promising area in greater detail.
Predicting Disease Trajectories in Affective Disorders through Combined Imaging and Immune Marker Analysis
Affective disorders (AD), encompassing major depressive disorder (MDD) and bipolar disorder (BD), affect more than 10% of the German population and are associated with significant individual suffering. Despite their prevalence, the mechanisms that influence long-term disease trajectories remain poorly understood.
The Collaborative Research Centre TRR 393 entitled ,Trajectories of affective disorders: Cognitive-emotional mechanisms of symptom changes’, founded in October 2024, aims to remedy this deficit. Researchers from Marburg and Münster want to
- Track relapse and remission trajectories in AD,
- Identify cognitive-emotional mechanisms and neurobiological correlates of acute symptom changes and
- Develop and test mechanism-based interventions.
Funded by the DFG through 2028, this project builds on foundational work at URTC, where we have studied long-term changes in brain structure, immune signatures, and environmental factors in affective disorders. Using machine learning and integrated neuroimaging and immunological analyses, we have shown that recurrent AD is linked to increased loss of grey matter volume. Childhood maltreatment and other risk factors also influence disease progression by altering cortical structure. In the DFG-funded research group „Neurobiology of affective disorders“ (FOR 2107) we are combining longitudinal imaging and immunological data to predict disease outcomes.
To implement these projects, some of the organisations involved have been working together on an interdisciplinary basis for more than a decade. During this time, valuable resources have been created:
- Detailed characterisation of samples from thousands of patients, which depict all aspects of CNS inflammation and also make the course of the disease visible at a molecular level,
- An infrastructure for conducting clinical trials at all stages, which was partially established together with the Medical Faculty of Münster (Early Clinical Trial Unit)
- The knowledge and methods to analyse various biological compartments and make them usable for studies
- A working group on stem cell technologies funded by the URTC (working group leader Yotam Menuchin-Lasowski). The methods used there allow interactions between different cell types - such as neurones, glia and peripheral immune cells - to be visualised. This means that inflammatory processes in the central nervous system can be analysed more precisely than ever before.
- Close collaboration with experts in the field of artificial intelligence and computational biology to analyse large multimodal data sets.
- A junior endowed professorship (W1) for systems biology in neuroinflammatory diseases (Prof. Christian Thomas)