The role of the Crumbs2 protein in podocytes
Cell polarity refers to the ordered but asymmetric distribution of proteins and membrane components within a cell. It forms the basis for many essential cellular processes. Both cell polarization and the associated formation of cell–cell junctions (tight junctions and adherens junctions) are regulated by evolutionarily conserved multiprotein complexes.
One such complex is the Crumbs complex, which consists of the core components Crumbs, Pals1 (see prroject Pals1), and Patj, and primarily regulates the establishment of apical membranes within the plasma membrane.
In humans, there are three different Crumbs genes (CRB1, CRB2, and CRB3). All Crumbs proteins share a common structure: an N-terminal extracellular domain (ECD), a transmembrane domain (TMD), and a C-terminal intracellular domain (ICD). The most significant differences lie in the ECD. In CRB1 and CRB2, the ECD is large and contains several EGF-like and Laminin G domains, while the much smaller CRB3 proteins lack most of the ECD.
In the kidney, CRB2 and two splice variants of CRB3 — CRB3A and CRB3B — are expressed. Both CRB3 isoforms are found in all renal epithelial cells, whereas CRB2 is primarily expressed in glomerular podocytes. These are highly polarized, post-mitotic cells that form part of the glomerular filtration barrier. Between their extensively branched cellular processes, podocytes form unique cell–cell junctions, known as slit diaphragms, which are exclusive to this cell type.
Recently, it was shown that specific mutations in the human CRB2 gene are associated with severe damage to the renal filtration barrier. This suggests that CRB2 has podocyte-specific functions that are essential for maintaining the integrity of this barrier.
The goal of our DFG-funded project (DFG WE 2550/2-2; project number 469263956) ) is to analyze in detail the cell biological functions of CRB2 and their significance for podocytes. To achieve this, we use various cell systems in combination with advanced microscopy techniques. The project is conducted in close collaboration with the research group of Prof. Ulrich Kubitscheck (Biophysical chemistry, University of Bonn).
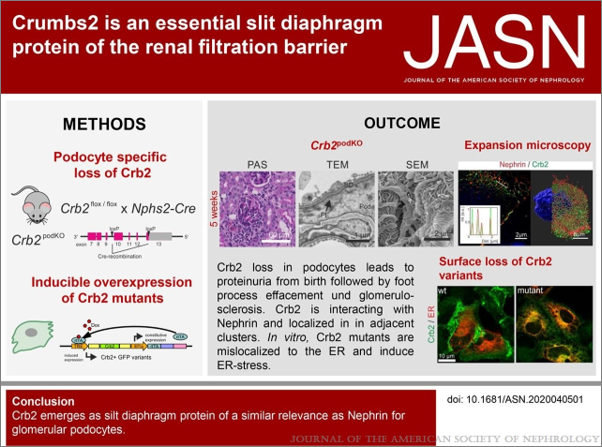
Möller-Kerutt, Annika et. al., JASN, May 2021
(Journal of the American Society of Nephrology 32-5,p 1053-1070; doi: 10.1681/ASN.2020040501)

