Research Topics
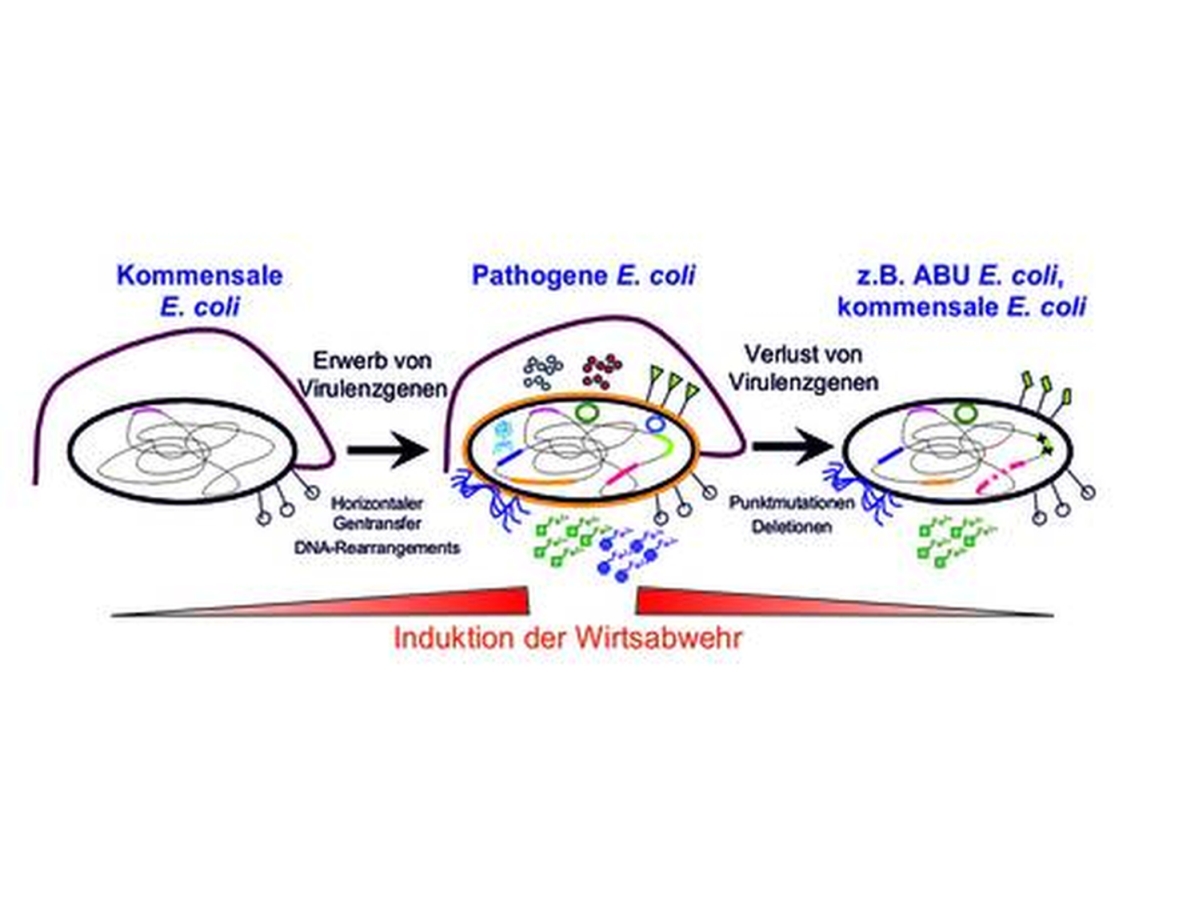
What distinguishes commensal and pathogenic Escherichia coli? Which bacterial traits are required to cause symptomatic or asymptomatic infection? How do bacterial pathogens change during the course of an infection?
To address these questions, we use Escherichia coli as a model organism, because this species comprises non-pathogenic, commensal as well as extraintestinal pathogenic E. coli (ExPEC) and intestinal pathogenic E. coli (IPEC) variants.
Whereas an IPEC infection causes diarrhoea, ExPEC mainly causes urinary tract infection (the most frequently occurring bacterial infection in industrial countries) as well as newborn meningitis. In addition, ExPEC are important nosocomial pathogens and causative agents of septicaemia. The increasing number and the spread of (multi-)resistant E. coli variants represent a major problem regarding the treatment of E. coli infections.
Using functional and comparative genomic analyses, we characterize virulence and fitness-associated genes of ExPEC and selected IPEC pathotypes. We study fundamental mechanisms of bacterium-host interaction which contribute to host colonization or the establishment of symptomatic or chronic E. coli infections.
In this context we are also interested in individual differences in genome content and structure which may contribute to the development of improved diagnostic, therapeutic or preventive approaches against ExPEC infection. Furthermore, we study differential regulation of gene expression, metabolic traits and interaction strategies with eukaryotes which may distinguish commensals from pathogens or different E. coli pathotypes.
Another focus is on the characterization of microbial genome plasticity and its impact on (i) the evolution and of antibiotic resistances, (ii) microbial pathogenicity, and (iii) adaptation (microevolution) in different niches and environments.
To address these questions, we use Escherichia coli as a model organism, because this species comprises non-pathogenic, commensal as well as extraintestinal pathogenic E. coli (ExPEC) and intestinal pathogenic E. coli (IPEC) variants.
Whereas an IPEC infection causes diarrhoea, ExPEC mainly causes urinary tract infection (the most frequently occurring bacterial infection in industrial countries) as well as newborn meningitis. In addition, ExPEC are important nosocomial pathogens and causative agents of septicaemia. The increasing number and the spread of (multi-)resistant E. coli variants represent a major problem regarding the treatment of E. coli infections.
Using functional and comparative genomic analyses, we characterize virulence and fitness-associated genes of ExPEC and selected IPEC pathotypes. We study fundamental mechanisms of bacterium-host interaction which contribute to host colonization or the establishment of symptomatic or chronic E. coli infections.
In this context we are also interested in individual differences in genome content and structure which may contribute to the development of improved diagnostic, therapeutic or preventive approaches against ExPEC infection. Furthermore, we study differential regulation of gene expression, metabolic traits and interaction strategies with eukaryotes which may distinguish commensals from pathogens or different E. coli pathotypes.
Another focus is on the characterization of microbial genome plasticity and its impact on (i) the evolution and of antibiotic resistances, (ii) microbial pathogenicity, and (iii) adaptation (microevolution) in different niches and environments.