INF2 and focal segmental glomerulosclerosis (FSGS)
INF2 and focal segmental glomerulosklerosis (FSGS)
Podocytopathies range from rare genetic diseases, such as congenital nephrotic syndrome, to common acquired conditions, such as hypertensive nephropathy. Focal segmental glomerulosclerosis (FSGS) is a histologically defined podocyte disease with both genetic and acquired causes. In FSGS, podocyte damage occurs, leading to foot process effacement, proteinuria, and progressive podocyte loss, ultimately resulting in glomerulosclerosis.
Podocytes form the most selective component of the glomerular filtration barrier. They have primary foot processes that branch into numerous interdigitating secondary foot processes, creating a network surrounding the glomerular capillaries. These secondary foot processes are separated by the filtration slit, which is bridged by the so-called slit diaphragm—a specialized cell–cell junction formed by an interacting protein network. Proteins such as nephrin, podocin, and the intracellular components CD2AP and ZO-1 form the slit diaphragm complex in podocyte foot processes (H. Pavenstädt et al., Physiol Rev, 2003).
The mechanical forces acting on podocytes are counterbalanced by the actin cytoskeleton in their foot processes. Almost all podocyte diseases are characterized morphologically by a foot process effacement associated with actin cytoskeleton remodeling. In recent years, approximately 40 monogenic mutations have been identified that lead to FSGS. Some of these monogenic podocytopathies are caused by mutations in actin-associated proteins expressed in podocytes, such as ACTN4, INF2, MYO1E, MYH9, and ANLN. These mutations lead to varying disruptions in podocyte function, many of which remain poorly understood.
Mutations in INF2 are the most common cause of autosomal dominant FSGS (C. Schell und T.B. Huber, J Am Soc Nephrol, 2017; D. Feng et al., Am J Physiol, 2018). The group of R. Wedlich-Söldner recently demonstrated that INF2 plays a crucial role in calcium-mediated actin reorganization (CaAR) in cells (P. Wales et al., Elife, 2016). The group of R. Wedlich-Söldner recently demonstrated that INF2 plays a crucial role in calcium-mediated actin reorganization (CaAR) in cells (S. Bayraktar et al., J Am Soc Nephrol, 2020). This actin reorganization may serve as a useful readout for systematically analyzing INF2-related mutations.
In the coming years, we aim to investigate whether INF2-related repair processes in podocytes can be stimulated and to determine the in vivo function of INF2 in podocyte biology. To this end, we have generated a podocyte-specific INF2 knockout mouse.
The Lasp1 protein: a novel link between slit diaphragm and actin cytoskeleton dynamics in podocytes
The Lasp1 protein: a novel link between slit diaphragm and actin cytoskeleton dynamics in podocytes
Members of the nebulin family regulate actin filament stability and function in muscle. The two smallest family members, Lasp1 and Lasp2, are also expressed in non-muscle tissues. Lasp1 is a multi-domain protein containing an N-terminal LIM domain, two actin-binding nebulin modules, a linker region, and a C-terminal SH3 domain. LIM domains are protein–protein interaction motifs composed of two zinc-coordinating modules (E. Butt und D. Raman, Front Oncol, 2018).
We recently demonstrated that Lasp1 is expressed at the slit diaphragm in podocytes and anchored to the actin cytoskeleton. Interestingly, Lasp1 interacts with the slit diaphragm protein CD2AP. CD2AP knockout results in a redistribution of LASP1 expression in podocytes. In collaboration with Prof. M. Krahn's group, we showed that Lasp1 plays an important role in maintaining slit diaphragm integrity in nephrocytes (C. Lepa, FASEB J, 2020). In the coming years, we will investigate the role of Lasp1 in regulating actin cytoskeleton dynamics in podocytes, both in vitro and in vivo.
The role of the Rab7 GTPase in podocyte function
The role of the Rab7 GTPase in podocyte function
An imbalance between endocytosis and autophagy plays a key role in the pathogenesis of kidney diseases. We hypothesize that late endolysosomal transport plays specific roles in podocyte function. To test this, we knocked out the GTPase Rab7 — a key enzyme involved in late endo- and autolysosomal trafficking — specifically in mouse podocytes. These podocyte-specific Rab7 knockout mice develop severe podocyte injury and proteinuria.
Using cell biological techniques, we aim to elucidate the mechanisms that lead to the development of podocytopathy. This project will provide critical insights into the role of late endolysosomal trafficking in maintaining podocyte function.
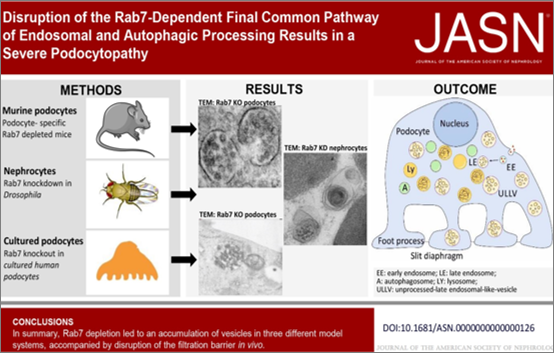
Disruption of the Rab7-Dependent Final Common Pathway of Endosomal and Autophagic Processing Results in a Severe Podocytopathy
Vöing, Kristin et. al., JASN, July 2023
(Journal of the American Society of Nephrology 34Molecular renal mechanisms of ischemic preconditioning
Molecular renal mechanisms of ischemic preconditioning
Acute kidney injury (AKI) is a common clinical complication associated with high mortality and currently lacks specific therapeutic options. Within the framework of the Clinical Research UnitKFO 342 "Organ Dysfunction During Systemic Inflammation Syndromes" (DFG-Projektnummer 414847370), and in collaboration with Prof. Dr. M. Meersch (Department of Anesthesiology, Intensive Care, and Pain Medicine), our project"The effects of DAMPs and remote ischemic preconditioning on the prevention of acute kidney injury (P7)" die renalen Schutzmechanismen nach einer ischämischen Präkonditionierung.
investigates renal protective mechanisms following ischemic preconditioning.
Ischemic preconditioning involves repeated interruption of blood flow to the arm using a blood pressure cuff, interspersed with reperfusion phases. This method is known as remote ischemic preconditioning (RIPC).
Clinical research from the Department of Anesthesiology has shown that RIPC can reduce the incidence of AKI in certain high-risk groups (A. Zarbock et al., JAMA, 2015). We aim to understand the molecular mechanisms by which muscle-derived proteins protect the kidney.
One hypothesis is that HMGB1, released from muscle during RIPC, protects renal tubules by inducing a cell cycle arrest. Using molecular and cell biology techniques, we aim to clarify how RIPC influences cellular signaling across different microdissected tubular segments of the kidney during acute kidney injury.
source: KFO342

