Our group is interested in the molecular and pathogenic mechanisms of rare genetic kidney diseases. Driven by the rapid advancements in modern DNA sequencing technologies (next-generation sequencing), genetic disorders have increasingly come into focus in biomedical research in recent years. The particular strength of this approach lies in the ability to identify novel disease mechanisms directly from patient samples, without the need for prior biological knowledge to generate hypotheses. This allows for the discovery of entirely new pathogenic mechanisms, which have revolutionized our understanding of kidney diseases and contributed to the development of new therapeutic strategies.
Our current projects focus on two groups of genes, both of which cause genetic podocyte diseases:
The KEOPS complex, consisting of the components LAGE3, OSGEP, TP53RK, and TPRKB, is an evolutionarily highly conserved, enzymatically active multi-protein complex with essential functions in protein biosynthesis. Affected patients present with an early-onset, syndromic disease characterized by malformations of the central nervous system and a rapidly progressive loss of kidney function (Galloway-Mowat syndrome).
Mutations in various nucelar pore proteins, which form a selective transport channel between the cell nucleus and the cytoplasm, cause a congenital podocyte disease in affected children. This condition is characterized by the onset of nephrotic syndrome and leads to a chronically progressive kidney disease already in early childhood.
Disease mechanisms of podocyte injury caused by mutations in genes encoding proteins of the tRNA modifying KEOPS complex
Disease mechanisms of podocyte injury caused by mutations in genes encoding proteins of the tRNA modifying KEOPS complex
The KEOPS complex catalyzes a specific post-transcriptional modification of tRNA molecules. This function is evolutionarily highly conserved but has remained poorly understood to date. It was only through our genetic investigations that a connection to human disease became apparent, leading to the hypothesis that tRNA modifications play a particularly important role in podocytes.
To gain new insights into the molecular pathomechanisms of podocyte injury based on our genetic findings, we conducted further studies using established cell culture systems. These studies yielded several interesting results:
First, we identified endoplasmic reticulum (ER) stress as a cause of podocyte injury and a trigger of apoptosis in podocytes. This finding is of particular interest because ER stress and the associated activation of the unfolded protein response (UPR) represent a promising target for novel, targeted therapies against podocyte damage.
Second, as a consequence of KEOPS loss of function, we observed a reduced rate of protein biosynthesis. Since podocytes are not secretory cells, this raises the intriguing question of why this particular cell type is especially vulnerable to disturbances in protein biosynthesis—an avenue that opens up interesting perspectives for further research.
In addition, we identified a series of secondary phenomena that underscore the central role of the KEOPS complex in podocytes, such as genomic stress with activation of the DNA damage response cascade and disturbances in actin homeostasis.
In summary, we discovered a previously unknown pathogenic mechanism of podocyte injury, characterized the resulting cellular defects, and identified a promising new target for future therapeutic interventions.
Our current work employs various cell culture systems, transcriptome analyses, and relevant animal models (particularly mouse and Drosophila melanogaster), to further elucidate the pathogenesis of KEOPS-associated podocyte diseases (DFG project number 391152220).
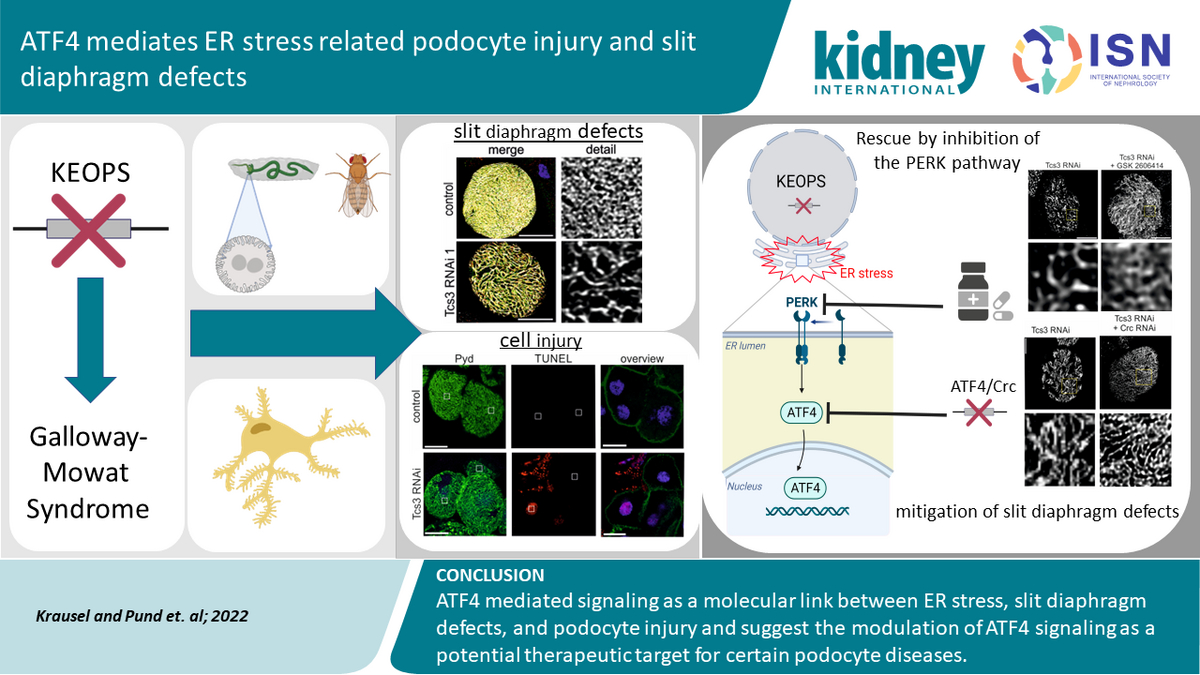
The transcription factor ATF4 mediates endoplasmic reticulum stress-related podocyte injury and slit diaphragm defects
Krausel, Vanessa und Pund, Lisanne et.al., Kidney International, December 2022
(Kidney International, 103-5, p872-885,
https://doi.org/10.1016/j.kint.2022.11.024)Punktmutationen in Proteinen der Kernpore, wie beispielsweise des Nukleoporins NUP93, als Ursache des vererbten nephrotischen Syndroms
Point mutations in nuclear pore proteins, such as the nucleoporin NUP93, as a cause of inherited nephrotic syndrome
In genetic studies, we identified mutations in various structural proteins of the nuclear pore complex (NUP85, NUP93, NUP107, NUP133, NUP160, and NUP205), which cause early-onset and progressively worsening kidney disease in affected children. This disease is characterized by podocyte injury, nephrotic syndrome, and chronic kidney failure.
The nuclear pore is a selective transport channel between the nucleus and the cytoplasm and represents the only connection between these compartments in eukaryotic cells. The spatial separation of transcription and translation—two consecutive steps in the conversion of genetic information into proteins—is essential for enabling cells to respond to external stimuli and adapt to changing environmental conditions.
This essential and universal function of nuclear pores in all nucleated cells contrasts with our genetic findings, which reveal a kidney-specific phenotype in affected patients. We therefore hypothesize that certain NUP proteins may have an additional, cell type–specific function in podocytes that is independent of their canonical role within the nuclear pore. Alternatively, it is conceivable that podocytes, due to their post-mitotic nature, are particularly vulnerable to disturbances in nuclear pore function.
In our project, we aim to resolve this apparent contradiction and gain a better understanding of the molecular disease mechanism underlying nuclear pore–associated podocyte injury. To this end, we have developed cell culture systems that allow for inducible knockout of NUP proteins as well as live cell imaging to visualize nuclear transport in real time. Additionally, we are studying mouse models that replicate the human phenotype and are therefore well suited for translational research.

