APOL1 as a risk factor for kidney diseases
Subspecies of the single-celled parasite Trypanosoma brucei cause African sleeping sickness (also known as African trypanosomiasis). This tropical disease is transmitted by the tsetse fly and occurs primarily in various tropical regions of Africa. Human blood serum contains a factor that normally protects people from this disease. In 2003, this factor was identified as APOL1 (short for Apolipoprotein L1). APOL1 — which occurs only in humans and a few other higher primates — is both necessary and sufficient to trigger this trypanolytic effect.
A co-evolutionary process between the SRA resistance gene (serum resistance-associated protein) of the trypanosomes and the trypanolytic factor APOL1 has led to the emergence of two APOL1 gene variants (G1 and G2), which are relatively common among African Americans and populations in West Africa.
In 2010, two research groups independently demonstrated that these specific APOL1 variants are associated with an increased risk of HIV-associated nephropathy (HIVAN). Subsequent studies have repeatedly confirmed this link. At the same time, it became evident that individuals who are homozygous for these APOL1 variants also carry a higher risk for other kidney diseases. Kidney diseases associated with APOL1 are also referred to as APOL1-mediated kidney diseases (AMKD).
However, not every carrier of APOL1 risk variants develops disease. It is therefore assumed that in addition to the genetic predisposition, at least one other factor (a "second hit") is necessary to trigger kidney disease. Elevated cytokine concentrations — such as interferons — or viral infections (particularly HIV or COVID-19) may play a particularly important role in this context.
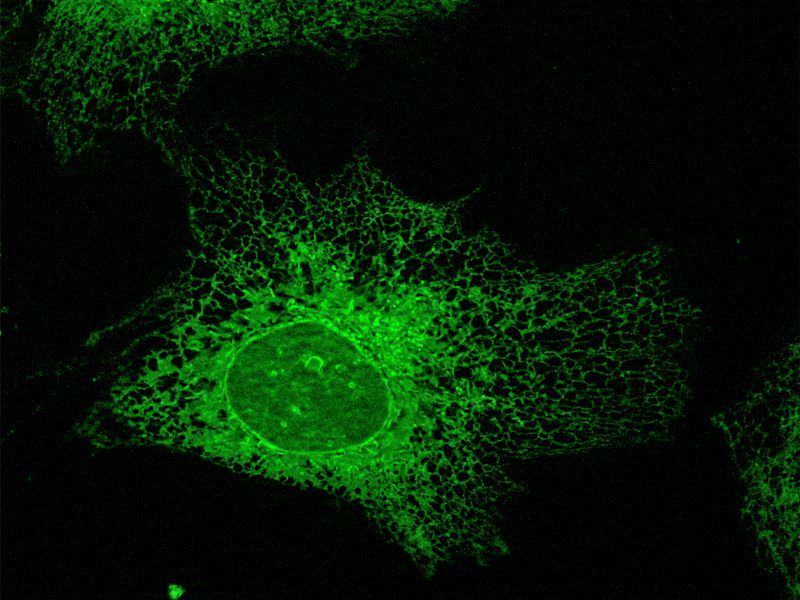
Research on APOL1 thus lies at the intersection of parasitology, evolutionary biology, infectious diseases, and nephrology. On a molecular level, however, it remains largely unclear what exact function APOL1 normally serves in the cell, and how it differs from other members of the APOL gene family. In previous work, we were able to show that APOL1 is primarily localized to the endoplasmic reticulum (ER), and that increased expression of the APOL1 risk variants leads to energy depletion in cells.
In our project funded by the German Research Foundation (DFG) (WE 2550/3-1 & 3-2; project number 324783603), we are now using primarily cell biological approaches to more precisely analyze the impact of APOL1 on ER-associated functions (e.g., biosynthesis, protein secretion).

