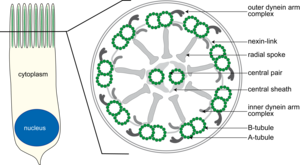
Figure1: Sketch of a ciliated respiratory epithelial cell (left) and schematic cross section (right)at the midsection of cilia.
For the immunofluorescence (IF) diagnosis of PCD, various components (proteins) of cilia structure are stained by specific antibodies. The cross section of a normal respiratory tract cilium shows the typical anatomy of the axoneme, which consists of 9 peripheral microtubule doublets that surround 2 centrally located single microtubules (9 +2 structure). The peripheral microtubule doublets are interconnected to each other by inner and outer dynein arms and to the central microtubules by radially oriented spokes (Figure 1). The outer and inner dynein arms are protein complexes of various dynein chains and associated proteins. A mutation in one of the genes that encodes a component of the dynein arms often results in a total loss of dynein arm structure. PCD diagnosis is also evident in electron microscopy (EM), although detection of the inner dynein arms, due to their poor contrast, is less robust than by detection using IF methods. Additionally, biopsy material for EM must be obtained by bronchoscopy, which requires anesthesia, whereas IF biopsy material is obtained by simple nasal brushing and is less burdensome to the patients.
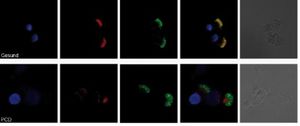
Figure 2: Immunofluorescence microscopy of respiratory epithelial cells. Representation of a constituent of the outer dynein arms (red) and of the tubule (green). The nucleus is stained blue. The yellow color indicates the spatial proximity (co-localization) of the proteins. From left to right: Nuclear staining (blue), DNAH5 (red) and acetylated alpha-tubulin (green), fusion (yellow = colocalization), transmitted light. Top with a healthy subject, down from a PCD patient with absent outer dynein arm.
The nasal swab is performed with a small brush, which is inserted a few inches deep into the nasal cavity to slough off respiratory epithelial cells. This biopsy is relatively easy to perform, takes only seconds, and causes no patient discomfort except perhaps sneezing. The epithelial cells are spread on slides and prepared for IF microscopy – the preparative staining takes several hours and is schematically shown in Figure 3. First, the sample is fixed on the slide and treated to prevent nonspecific antibody binding (blocking). Then primary antibody specific to the protein of interest (black hexagon) is added (I) to the slides, and then washed briefly to remove excess, unbound primary antibody. Thereafter, the slides are incubated with a fluorescently labelled secondary antibody (II) that binds specifically to the primary antibody, and also washed briefly to remove excess, unbound secondary antibody. Finally, the nuclei are directly labelled with a dye, and the samples are embedded in a special medium for preservation. Then the biopsy material can be observed under the immunofluorescence microscope. The dye is excited by a laser (blue wavy line) and emits a specific wavelength that is detected by the device. The measured data can be used as single staining and, in case of multiple colors, are displayed as a calculated superposition. The detection of the outer dynein arms required specific anti-DNAH5 antibodies, which were produced by our group and allowed us to develop and establish this diagnostic method. The detection of the inner dynein arms is also now well established by IF microscopy, and detection of other components of the cilium by IF microscopy are in progress. A diagnosis by IF microscopy alone remains challenging and time consuming, and is thus considered in conjunction with the following common clinical symptoms to establish a diagnosis of PCD:
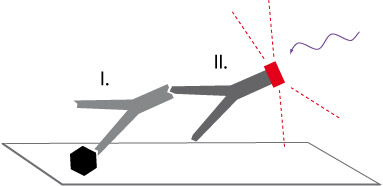
Figure 3: Immunofluorescence staining statement in the text above
- RDS-like image in the neonatal
- recurrent, severe infections of the respiratory tract
- chronic productive cough
- recurrent otitis media
- Development of bronchiectasis (in childhood and adolescence)
------------------------------------------------------------------------------------
- situs inversus and chronic respiratory disease (Kartagener syndrom)
The diagnosis is performed in our laboratory in Muenster. For further information and documentation, see the following links or contact us directly to our laboratory in Muenster, and we will send you a kit. We welcome additional participation in our genetic study of PCD.
Information and documents for immunofluorescence diagnosis (brush biopsy)
Minutes of the brush biopsy (the doctor)
Accompanying sheet for the brush biopsy
Link to our "genetic study"
Further Reading

