Research Overview
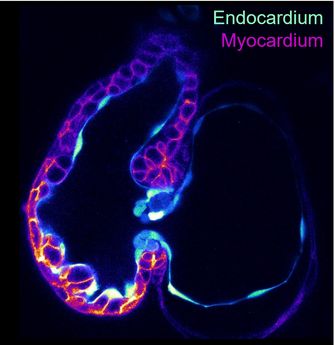
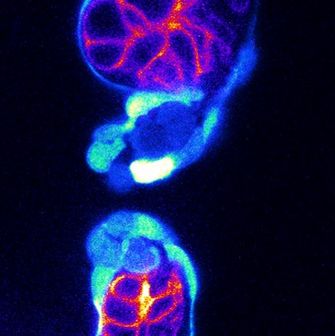
The contractile heart is the first organ that forms, with the contractile cardiomyocytes and the endocardium forming the first tissues of the heart. Without the endocardium, the heart does not form. Once formed, the endocardium protects the outer cardiomyocytes from blood flow, and gives rise to the cardiac valves, which prevent retrograde blood flow. Malformations of endocardial-derived structures lead to congenital heart defects, the leading cause of death by birth defects, emphasizing the importance of proper endocardial development. However, despite its importance, little focus has been given to elucidating the cellular and molecular processes that promote the formation and function of the endocardium compared to the cardiomyocytes. Notably, besides the specialized endocardial cells that form the cardiac valves, heterogeneity within endocardial subpopulations at the cellular, molecular, and functional levels is almost uncharacterized.
Using the zebrafish embryo as a model, the research group aims to elucidate the functional requirements and cellular and molecular processes that form the endocardium, with three broad objectives:
(1) Elucidate the cellular and molecular processes that form the cardiac valves, consisting of the outer lining of valve endothelial cells, and the inner fibroblast-like valve interstitial cells, both of which derive from endocardial cells. We are particularly focusing on the downstream targets of the transcriptional regulator Nfatc1, and its role in activating extracellular matrix genes in the valve interstitial cells. We are further delineating the function of the fibrillar matrix derived from the valve interstitial cells, and the basement membrane matrix derived from the valve endothelial cells.
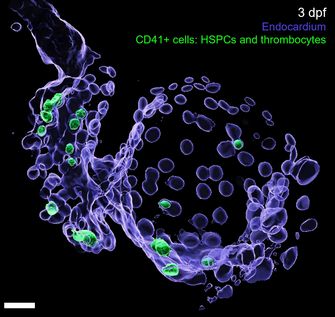
(2) Uncover the plasticity potential of the endocardial cells. During development and injury, endocardial cells have a high capacity to trans-differentiate and contribute to other cell types. Our data show that the endocardial cells contribute into the hematopoietic lineages, notably into hematopoietic stem and progenitor cells and erythrocytes. We are focusing on the role of these endocardial-derived hematopoietic and other cardiac cells during development and in response to injury, and on the molecular regulators that drive the contribution of endocardial cells into hematopoietic cells.
(3) Establish an in vivo model of cardiac infection. Damaged or defective endocardial-derived structures lead to a higher propensity of developing bacterial infection in the heart. We aim to establish a model to study the cellular and molecular processes that cause this infection, with a goal to identify mechanisms to prevent or eradicate cardiac infection.


