Special model: Induced pluripotent stem cells
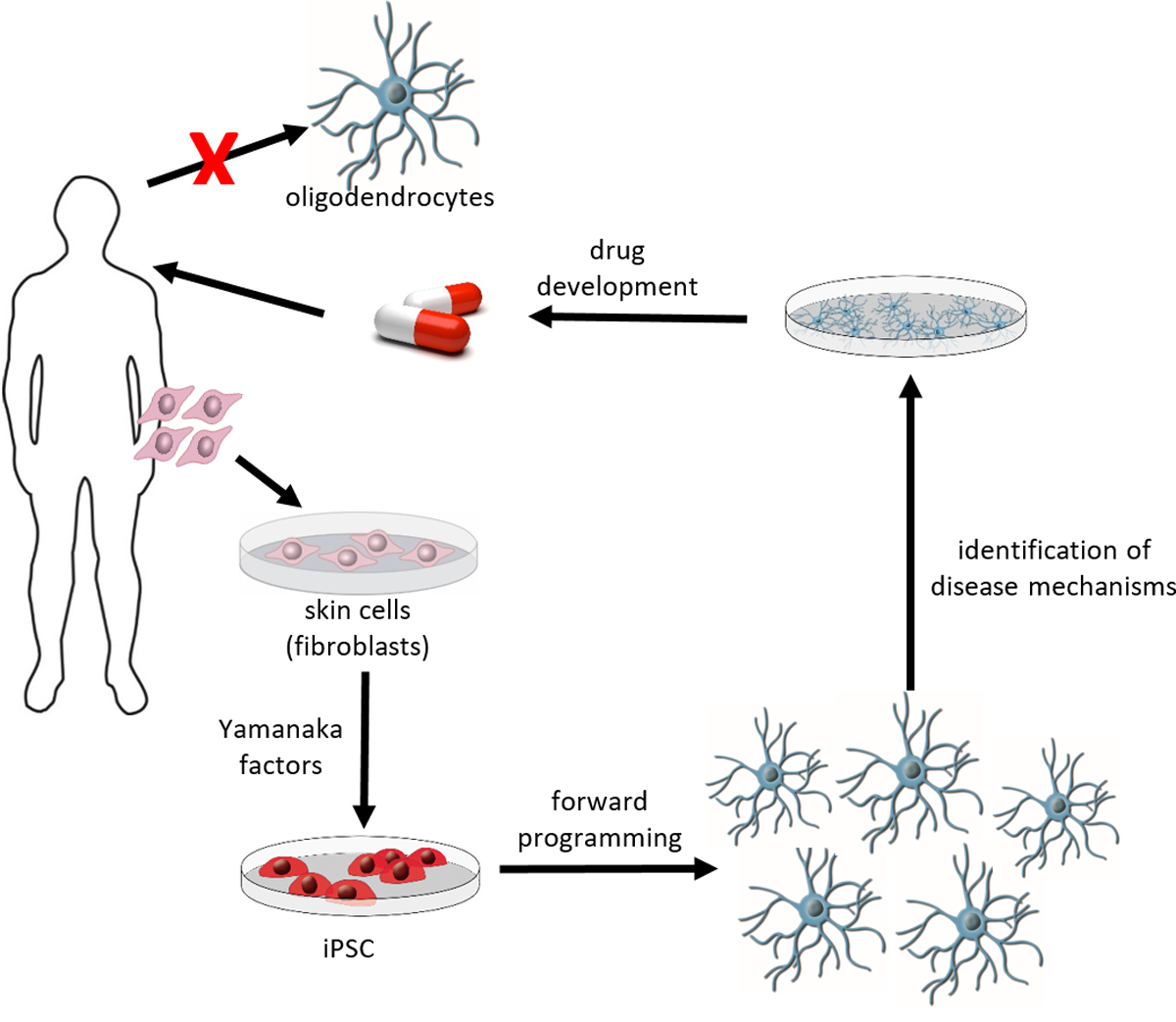
Diseases of the human central nervous system (CNS) represent a major challenge. The numbers of patients with diseases of the central nervous system are constantly increasing due to better health care and increasing life expectancy. Despite intensive research efforts in universities, research institutes and pharmaceutical industry the treatment options for many CNS diseases are still nonsatisfying. One reason for the limited success so far is the restricted availability of primary human CNS cells, such as neurons, astrocytes and oligodendrocytes. In 2006, a new technology was presented by Nobel Laureate Shinya Yamanaka to derive pluripotent stem cells from differentiated somatic cells without the need to isolate pre-implantation blastocysts. These induced pluripotent stem cells (iPSC) can be generated by introducing a set of pluripotency-associated transcription factors into differentiated somatic cells, which modify their epigenetic status leading to cellular reprogramming. As pluripotent cells, iPSC share main characteristics of embryonic stem cells, such as the ability for unlimited self-renewal and the potential to differentiate into cells of the three germ layers, mesoderm, endoderm and ectoderm. Since iPSC can readily be derived from human cells, including fibroblasts from skin biopsies, this technology has significant implications for regenerative medicine and biomedical research. Indeed, the derivation of iPSC from patients offers an invaluable opportunity to study disease pathogenesis in an in vitro-culture system on human cells at risk (e.g. oligodendrocytes or neurons from patients with myelin or neurodegenerative diseases), which is often difficult to accomplish in patients due to the unavailability of appropriate tissue (e.g. brain tissue of a patient).
Rapid, efficient and reproducible generation of human oligodendrocytes from iPSC
Oligodendrocytes are the myelin forming cells of the CNS and they play a key role in myelin related diseases including multiple sclerosis as well as neurodegenerative diseases or traumatic brain injury. Stem cell technologies, especially induced pluripotent stem cells are promising technologies to generate unlimited numbers of human oligodendrocytes. Access to large numbers of human oligodendrocytes will help to identify disease mechanisms and to develop new pharmaceutical treatment options. During recent years, we developed a protocol that allows the generation of human iPSC derived oligodendrocytes by forced expression of three transcription factors, namely SOX10, OLIG2 and NKX6.2 in iPSC derived neural progenitor cells (Ehrlich et al, PNAS 2017). Aim of this project is to optimize further the protocol to allow the fast, rapid and reliable production of human iPSC derived oligodendrocytes in high through put to facilitate and promote research of human CNS diseases and development of new treatment approaches in academia and pharmaceutical industry. This project is supported by the European Union and EFRE.NRW.