Themes
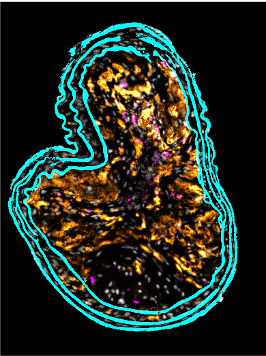
Neutrophils have traditionally been viewed as bystanders or biomarkers of cardiovascular disease. However, studies in the past decade have demonstrated the important functions of neutrophils during cardiovascular inflammation and repair. Our team we contributed to the understanding of neutrophil recruitment into atherosclerotic lesions and how they uphold key processes including the recruitment of monocytes and the expansion of the necrotic core. We have also studied how models of infection accelerate atherosclerosis and how neutrophils contribute to these processes. Finally, we not just aim at understanding physiology but also the generation of specific interference strategies to target neutrophil numbers, functional status and effector mechanisms.
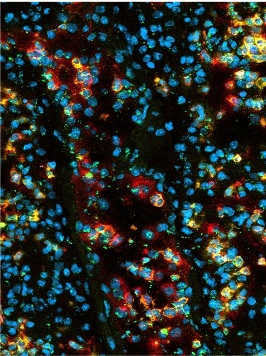
Neutrophil tissue infiltration frequently comes at the expense of host cell damage. Indeed, excessive neutrophil infiltration associated with tissue damage in a large variety of clinical settings including acute lung injury, myocardial infarction, stroke, as well as kidney and liver failure. Such collateral damage can result from the production of ROS, the release of cytotoxic granule proteins, and the release of NETs. In our studies we aim at deciphering how neutrophils kill host cells and how theses processes are altered upon metabolic challenge including hypercholesterolemia and hyperglycemia. In our histone-focused work we also generate and test peptide- and antibody-based neutralization strategies.
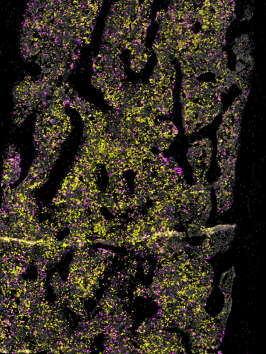
The essential immune functions and short lifespan of neutrophils demand their constant production in the bone marrow, a process that is referred to as granulopoiesis. With an estimated rate of production in humans of about 1011 neutrophils every day and the need to adapt to additional demands during conditions of stress, granulopoiesis is a highly regulated and energy-consuming process. In recent years, we have witnessed the emergence of defined marker sets to identify neutrophil maturation in the bone marrow. In our studies we study granulopoiesis is regulated in steady-state, acute inflammation and chronic metabolic stress. In addition, we work on defining processes of neutrophil mobilization from the bone marrow under these conditions.
Soehnlein Group 2024



