
Our Research
Our group addresses the important question of how vertebrate and invertebrate cells coordinate developmental behaviours with that of their neighbours. It is well established that one way to achieve this goal is that organizing cells secrete signaling molecules such as the Hedgehog (Hh) morphogens to instruct distant receiving cells in concentration-dependent or time-encoded manner. In vertebrates, the Hh family member Sonic Hh (Shh) is essential for patterning of the ventral neural tube, for specifying digit identities and for axon guidance. In the adult, Shh pathway activation has been implicated in maintaining the stem/cancer stem cell niche and in the progression of various cancers. Despite these important roles, various aspects of Hh solubilization, its transport and signaling function remain unclear. This is because Shh/Hh biosynthesis seems at odds with its established long-range signaling function: All Hh family members are secreted as dual-lipidated morphogens that firmly attach to the plasma membrane of the producing cell and thus render these signaling molecules insoluble. One advantage of working with the Hh signaling pathway is that all major players involved in Hh biosynthesis, release and reception are highly conserved in the fruit fly (Drosophila melanogaster). This allows us to develop hypotheses on Hh release and transport, test them initially in vitro using advanced biochemical and cell biology methods, and finally confirm their physiological relevance in vivo using the fly.
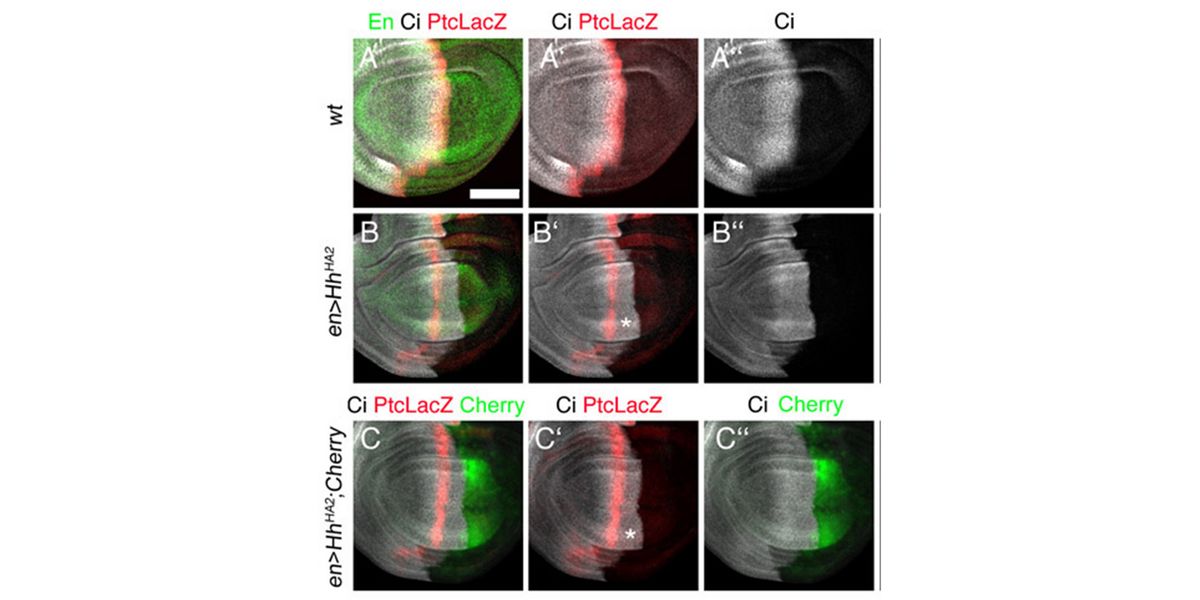
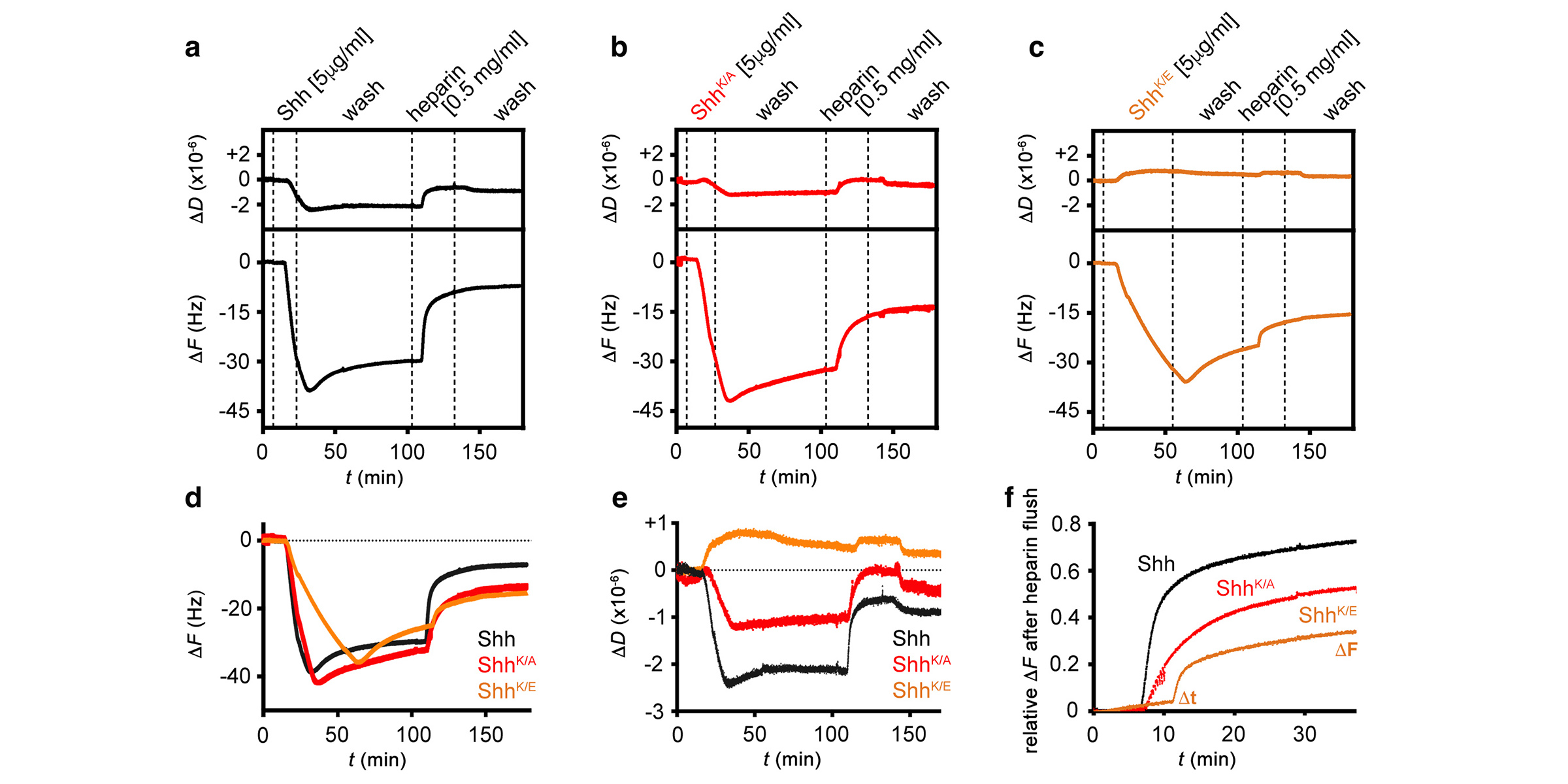
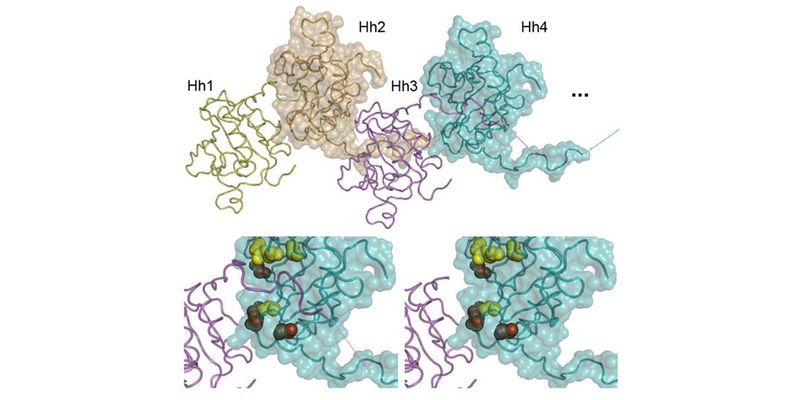
Using these combined methods, we have already established that Hhs are released from producing cells by proteolytic processing of their lipidated terminal Hh peptides (called shedding) in vitro (Dierker et al., 2009; Ohlig et al., 2011; Ohlig et al., 2012) and in vivo (Kastl et al., 2018; Schürmann et al., 2018, Manikowski et al., 2021). We also found that Hh shedding depends on accessory glycoproteins called heparan sulfate proteoglycans (HSPGs) (Grobe et al., 2005; Jakobs et al., 2016; Ortmann et al., 2015). HSPGs are extracellular proteins linked to highly anionic heparan sulfate (HS) glycosaminoglycan chains that bind Hhs and factors facilitating their regulated release (such as the soluble secreted glycoprotein Scube)(Jakobs et al., 2014; Jakobs et al., 2016; Jakobs et al., 2017, Manikowski et al., 2019). Most recently, we characterized the mechanistics of another established Hh/Shh release facilitator called Dispatched (Ehring et al., 2021, Manikowski et al., 2021, Ehring et al., 2022). Still, it remains only poorly understood how these different Hh release modulators steer morphogen function during development. Therefore, by using advanced microscopy in conjunction with biochemistry and fly genetics (using Hh-dependent eye- and wing development as read-outs), we currently characterize the molecular interplay between Hhs, the Hh sheddases, and sheddase modulators such as HSPGs, Scube, and Dispatched during Shh/Hh release (Gude, Ehring, Schulz, Kupich within the SFB1348 framework).
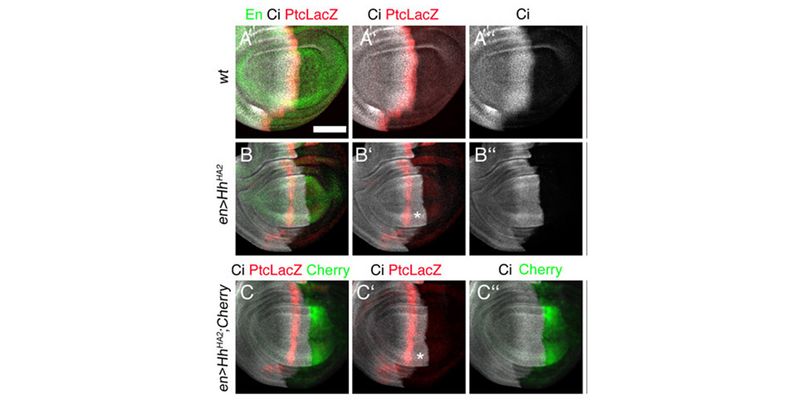
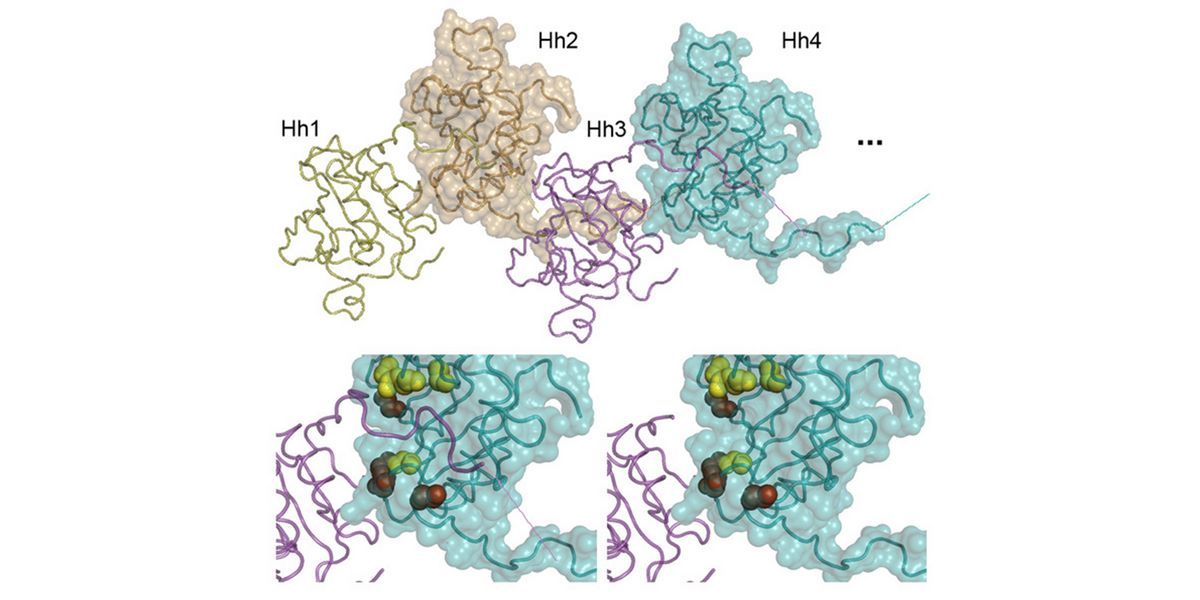
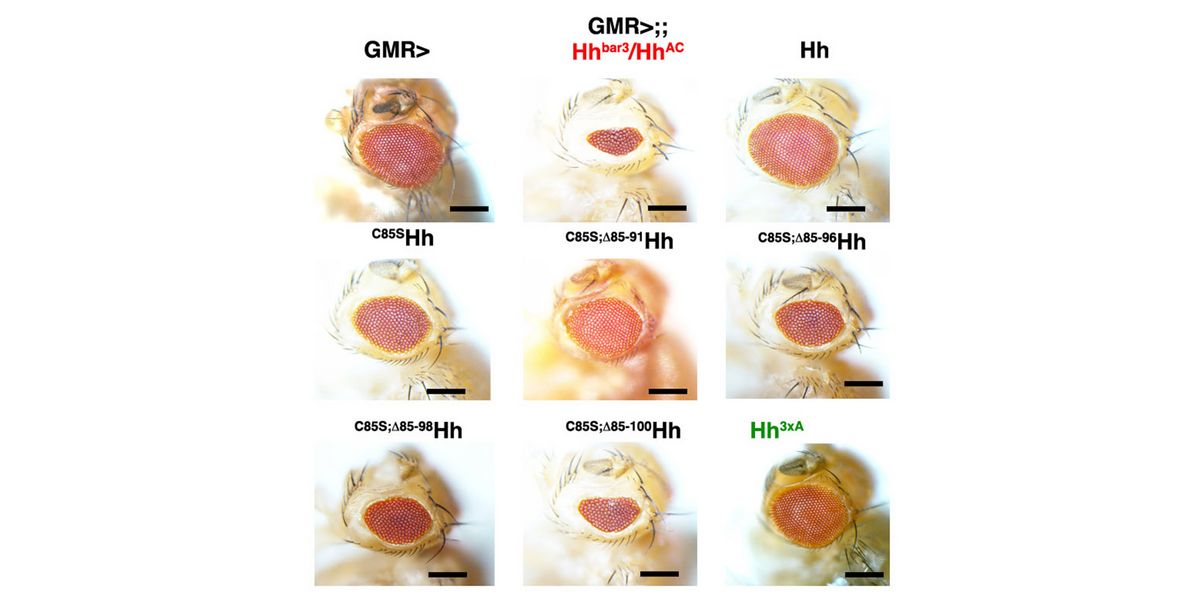
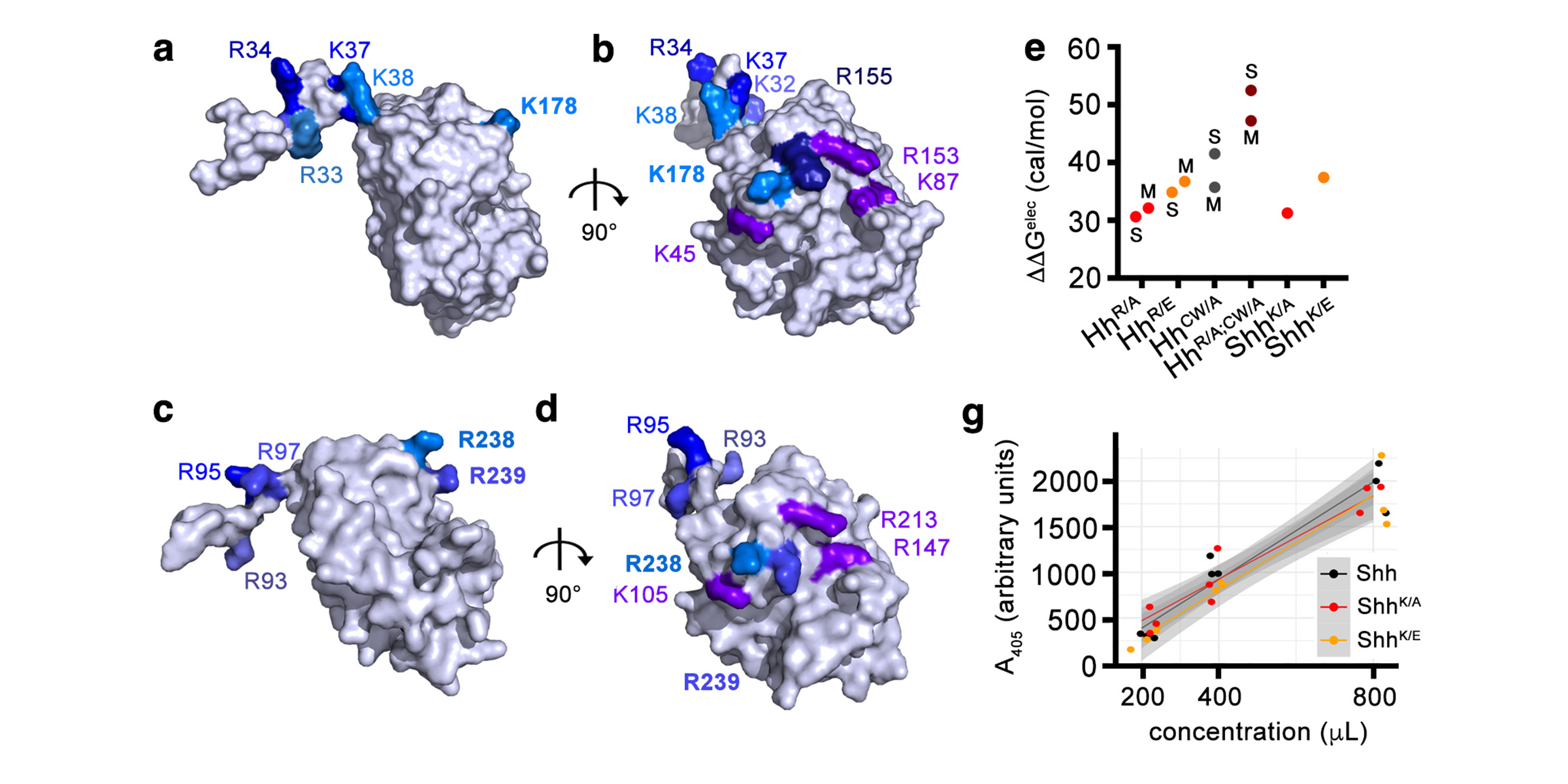
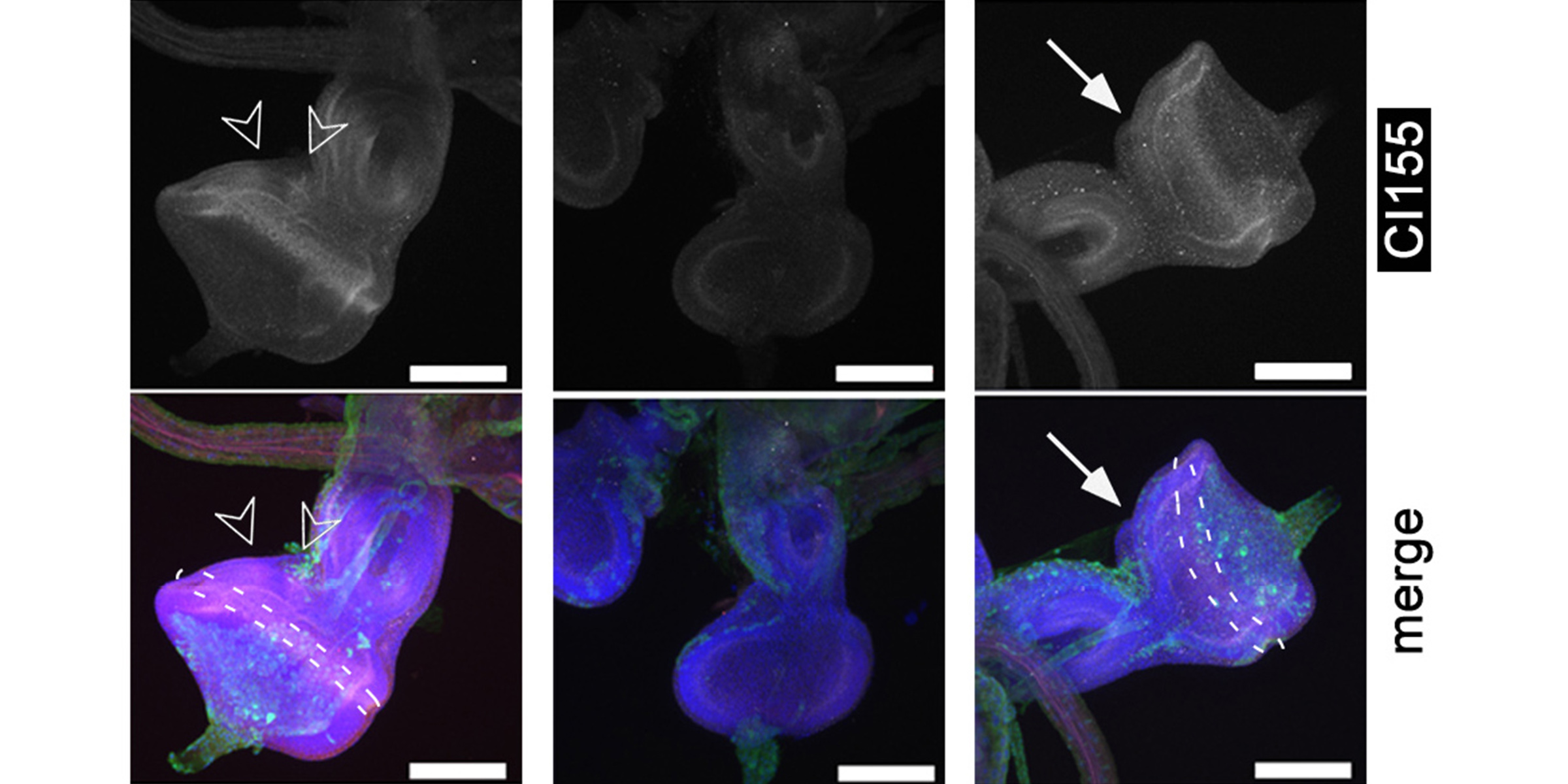
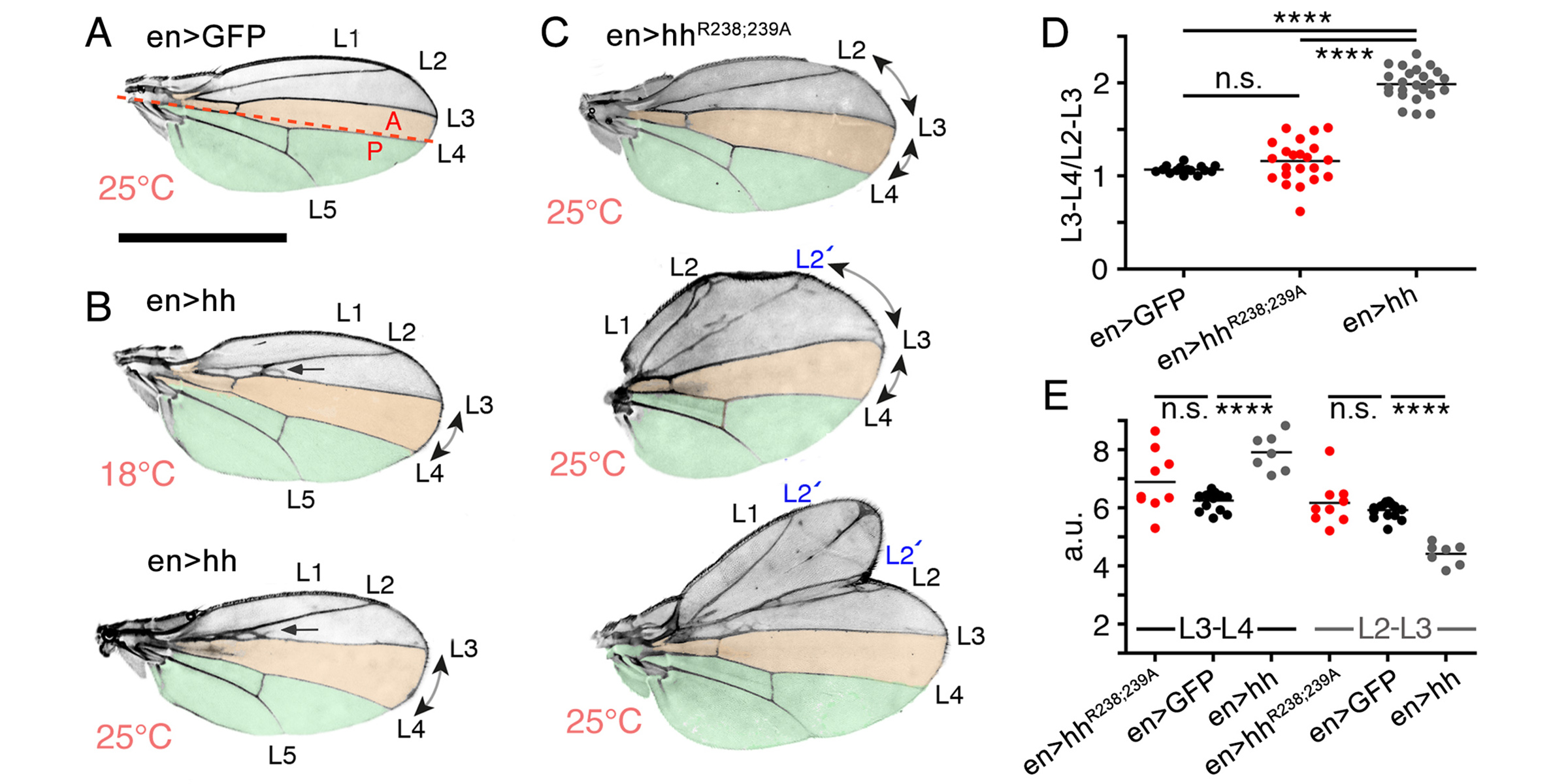
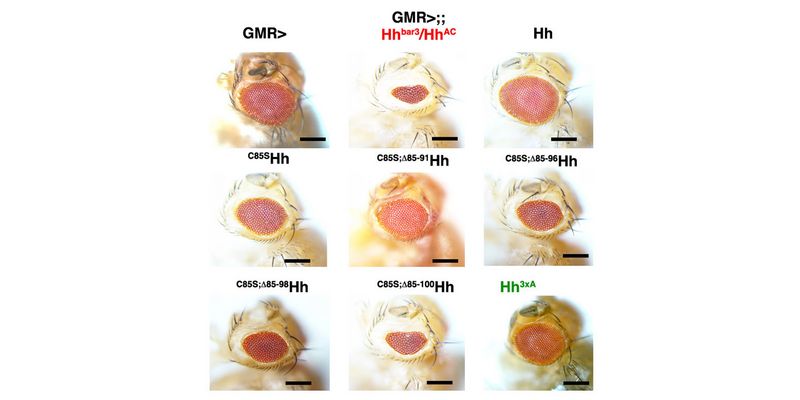
In a second line of DFG-funded research, we decipher mechanisms of Hh long-range transport in the Drosophila wing and eye disc by site-directed mutagenesis of HSPG-binding or calcium-coordinating Hh amino acids (Gude, Froese, Schulz, Kupich). Removal of selected Hh residues that bind to HSPGs was found to vastly increase its signalling range, resulting in striking patterning abnormalities such as mirror-image duplications of anterior wing tissue, while other residues have the opposite role. These findings provide a new mechanism to explain the essential role of HSPGs for accurate, defined and robust Hh signalling during development.

