Sex-specific impact of the NPS system on top-down control of fear behavior in mice
K. Jüngling
The life time prevalence for the development of anxiety or panic disorders is two to three times higher in women compared to men. Human and rodent research provides evidence that there are sex-specific differences in emotion regulation i.e. in fear extinction. In female rodents, attenuated fear extinction is regularly observed following classical fear conditioning. Recent data implicate an influence of the neuropeptide S system on fear extinction in a sex-dependent manner, where the functional polymorphisms of the neuropeptide S receptor (NPSR1) shape the time course of extinction predominantly in female mice. Although the amygdala has been identified as a crucial structure mediating these effects, nothing is known about the function of the NPS system in other highly fear-relevant circuits, which are known to mediate top-down control of emotion regulation, i.e. the medial prefrontal cortex (mPFC) and connected subcortical structures.
Although we have made progress understanding the role of the NPS system in the amygdala (Chauveau et al., 2012; Jüngling, Maren D. Lange, et al., 2015; Bengoetxea et al., 2021; Park et al., 2021), its function in mPFC circuits (i.e. the PL) and its contribution to observed sex-based differences in fear expression and extinction is almost unknown. Within this project, we will focus our research on the role of the NPS system and sex-differences within circuits mediating top-down control of fear expression and extinction. We will use a novel NPSR1-Cre driver line to specifically address components of the NPS-system in the medial prefrontal cortex, combine this mouse model with state-of-the-art methods (catFISH and chemogenetics) in conjunction with behavioral approaches to detect sex-specific differences in emotion regulation.
Here we aim to analyze 1) the electrophysiological properties of NPSR1-expressing neurons in the prelimbic region (PL) of the mPFC and their synaptic connections to subcortical (i.e. amygdala) regions, 2) identify their activation profile during fear memory retrieval and extinction using specific temporal and spatial distribution of immediate early gene mRNA, and 3) test their contribution to fear extinction via pathway-specific chemogenetic manipulation. We will compare these parameters between male and female mice of a novel transgenic NPSR1-Cre mouse line. Thereby, the project will contribute to the understanding of sex-specific differences in emotion regulation.
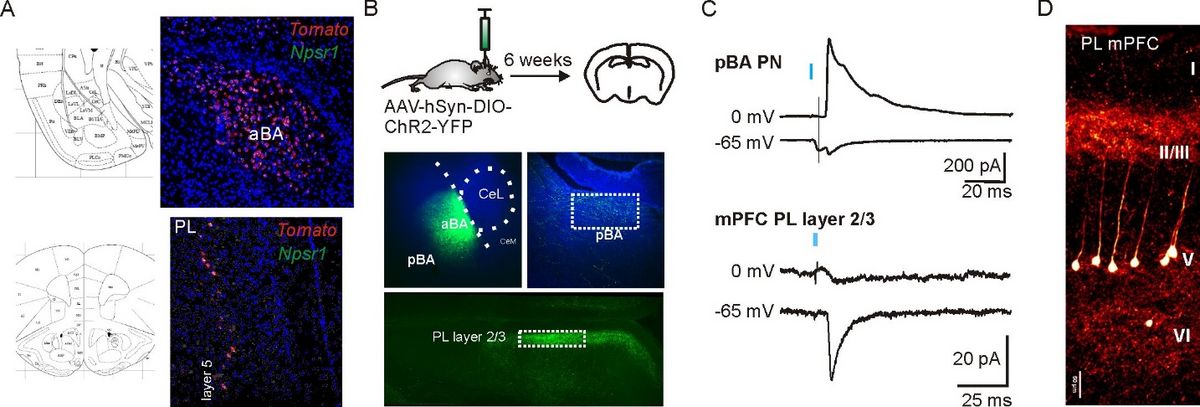
Characterization of a novel NPSR1-Cre driver mouse line. A) Expression of tdtomato (red) in nuclei of the amygdala (LA: lateral nucleus, aBA: anterior basolateral nucleus) and prefrontal cortex. Validation of Cre-dependent tdtomato expression using RNAScope to detect NPSR1 and tdtomato mRNA in double-transgenic mice. B) Pilot studies confirm functional Cre-dependent expression of ChR2-EYFP in aBA PNs six weeks after local stereotactic AAV-hSynI-DIO-ChR2-EYFP injection. EYFP-positive axons were detected in pBA and layer 2/3 of the prefrontal cortex (mainly prelimbic, PL, and anterior cingulate cortex, ACC). C) Light-evoked (470 nm) monosynaptic glutamatergic and large disynaptic GABAergic responses recorded from pBA PNs confirm our previous findings (Bengoetxea et al., 2021). In the PL, recordings from neurons in layer 2/3 revealed light-evoked glutamatergic inputs, but almost no GABAergic synaptic components. D) Confocal image of NPSR1-expressing neurons in layer 5 of the PL taken from a 300 µm thick coronal slice of a double transgenic mouse (NPSR1-Cre x tdtomato). NPSR1 neurons can be identified by the expression of tdtomato.

