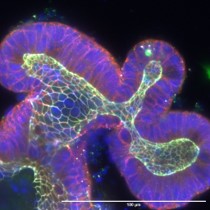
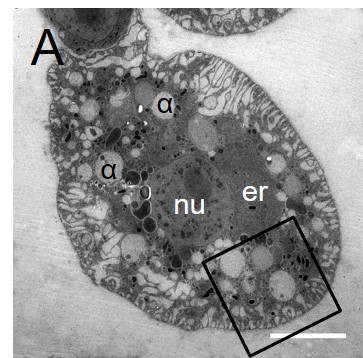
The tumour-suppressor LKB1 – Linking cell polarity to cell proliferation control
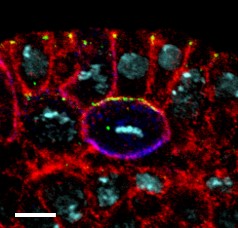
LKB1 is a master kinase, which regulates a variety of cellular pathways, thus influencing cell polarity, cell migration, cell proliferation and cellular metabolism. Mutations in lkb1 are associated with the Peutz-Jeghers-Syndrome, a rare dominant autosomal inherited cancer syndrome disease, in which patients develop benign gastrointestinal harmatomas and show a higher risk for intestinal and extraintestinal cancer. Notably, deletion of the lkb1 locus or downregulation of the protein expression is frequently found in various cancer specimen and -cell lines (with a few exceptions, (Cidlinsky et al., 2016)).
We found that LKB1 is not a constitutively active kinase but that its activity and protein stability is regulated by binding to the phospholipid phosphatidic acid (Dogliotti et al., 2017).In ongoing projects, we investigate the regulation of LKB1 and other kinases by phospholipids of the membrane using biochemistry approaches as well as super resolution microscopy and FRET-analyses. We further aim to elucidate the role of LKB1 in the regulation of cell polarity on the one hand and its impact on cell proliferation control and cellular metabolism on the other hand. We recently identified several potential interaction partners of LKB1 in a mass spectroscopy-approach, which are currently investigated for their role in cell polarity, cell proliferation and cellular metabolism.
Picture: Taken from Dogliotti et al. Nat Commun. 2017 Jun 26;8:15747. doi: 10.1038/ncomms15747, ©Creative Commons Attribution 4.0 International License.