Research interests
Work in our group focuses on the interaction between cellular membranes and the membrane-underlying cytoskeleton. Structural and dynamic aspects of such interactions and their relevance to a number of biological processes ranging from intracellular membrane/protein transport to the regulation of intercellular contacts are being investigated. A special emphasis is put on the role of Ca2+ ions transmitting as second messengers a variety of signals to the membrane associated cytoskeleton. Such Ca2+ signals are mediated through different Ca2+ binding proteins and our group is actively engaged in identifying and characterizing these Ca2+ mediators. Structural and functional characterization of Ca2+ binding proteins Intracellular Ca2+ signals are mediated through a number of Ca2+ effector proteins which upon binding of the divalent cation display specific changes in their conformation and/or properties. We are interested in understanding the structure and function of members of two multigene families of such Ca2+ effector proteins, the S100 proteins and the annexins.
Research interests
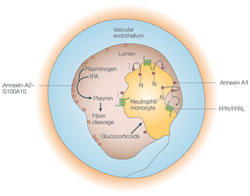
Work in our group focuses on the interaction between cellular membranes and the membrane-underlying cytoskeleton. Structural and dynamic aspects of such interactions and their relevance to a number of biological processes ranging from intracellular membrane/protein transport to the regulation of intercellular contacts are being investigated. A special emphasis is put on the role of Ca2+ ions transmitting as second messengers a variety of signals to the membrane associated cytoskeleton. Such Ca2+ signals are mediated through different Ca2+ binding proteins and our group is actively engaged in identifying and characterizing these Ca2+ mediators. Structural and functional characterization of Ca2+ binding proteins Intracellular Ca2+ signals are mediated through a number of Ca2+ effector proteins which upon binding of the divalent cation display specific changes in their conformation and/or properties. We are interested in understanding the structure and function of members of two multigene families of such Ca2+ effector proteins, the S100 proteins and the annexins.Mechanism of leukocyte extravasationInflammatory reactions are characterised by an extravasation of innate immune cells from the blood into the inflamed tissue. This process involves the polarization and directional migration of extravasating leukocytes and their specific passage through the endothelial lining of the vessel wall. We analyse molecular mechanisms controlling the directed migration of leukocytes and the formation of endothelial cell contact and their role in leukocyte transendothelial migration
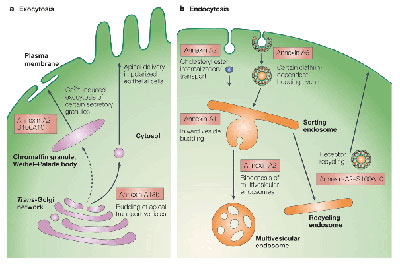
Membrane/protein transport in endocytosis and exocytosis
Vesicular traffic to (exocytosis) and from (endocytosis) the plasma membrane requires the concerted action of a multitute of accessory factory that often function in conjunction with the membrane-associated cytoskeleton. We are particularly interested in characterizing the role of membrane-associated proteins, e.g. of the annexin family, in regulating membrane transport and membrane-cytoskeleton interactions during endocytosis and exocytosis.
Vesicular traffic to (exocytosis) and from (endocytosis) the plasma membrane requires the concerted action of a multitute of accessory factory that often function in conjunction with the membrane-associated cytoskeleton. We are particularly interested in characterizing the role of membrane-associated proteins, e.g. of the annexin family, in regulating membrane transport and membrane-cytoskeleton interactions during endocytosis and exocytosis.