The (patho)physiological functions of the integrin inhibitors are analyzed in various cellular and physiological system, among them models of tumor progression and thrombosis (Eble JA 2005; Eble JA and Haier J 2006; Suzuki-Inoue K, et al. 2006; Fuller GLJ, et al. 2007; Hughes CE, et al. 2008; Rosenow F et al. 2008; Kerrigan AM, et al. 2009; Krähling H et al. 2009; Kusuma N, et al. 2012; Parguina AF, et al. 2012; De Cuyper IM, et al. 2013; Vivas-Ruiz DE, et al. 2013).
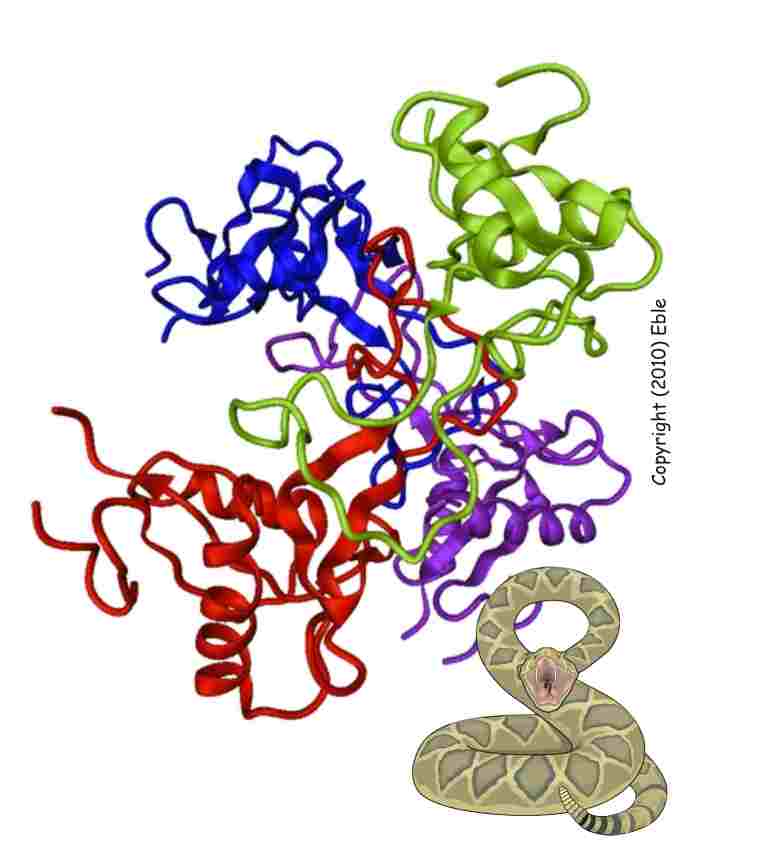
In parallel to their functions, the molecular structures of the integrin inhibitors are studied. The crystal structure, e.g. of the 21 integrin antagonist rhodocetin, provide insights into the molecular mechanism of integrin blockage and reveal potential lead structures to develop integrin-targeting pharmaceuticals (Watson AA, et al. 2008; Eble JA, et al. 2009; Bracht T et al. 2011).
The Eble lab appreciates the lively cooperations with various snake farms and venom research facilities in Brazil and Israel.
In addition to integrin inhibitors, snake venoms are a rich source for highly effective compounds interfering with a whole spectrum of physiological reactions, such as coagulation, thrombosis, regulation of cardiovascular integrity and function, of neuronal and muscular functions. As such, we could identify neuropilin-1 on endothelial cells as novel receptor for the subunit of rhodocetin leading to restructuring of the integrin-containing adhesome and stress fibers in endothelial cells (Niland S et al. 2013). But also CLEC-2 and GPIb were identified as potential target of C-type lectin-related proteins from snake venoms (Suzuki-Inoue K, et al. 2006; Fuller GLJ, et al. 2007; Watson A, et al. 2007; Kerrigan AM, et al. 2009; May F, et al. 2009; Hughes CE, et al. 2010; Pollitt AY, et al. 2010; Navdaev A et al. 2011; Séverin S et al. 2011; Parguina AF, et al. 2012; De Cuyper IM, et al. 2013).

