Prof. Dr. med. Astrid Jeibmann

Institut für Neuropathologie
Pottkamp 2
Tel.: +49-(0)251-83-56976
Fax.: +49-(0)251-83-56971
Email
Neurotoxicology
Clinical features and prognosis of major neurological diseases, such as Alzheimer´s, Parkinson´s, traumatic injury and stroke, are not only determined by mechanics, cell biology and genetics, but are strongly affected by the patient´s environment and behavior, such as social interaction, cognitive and physical activity. In addition, environmental and nutritive toxins (heavy metals, smoking, alcohol, illicit drugs, medical substances, chronic stress) may affect the course of neurological disease and often harm the brain per se. We are interested in mechanisms of these lifestyle effects on the brain using rodent and invertebrate models as well as human brain tissue.
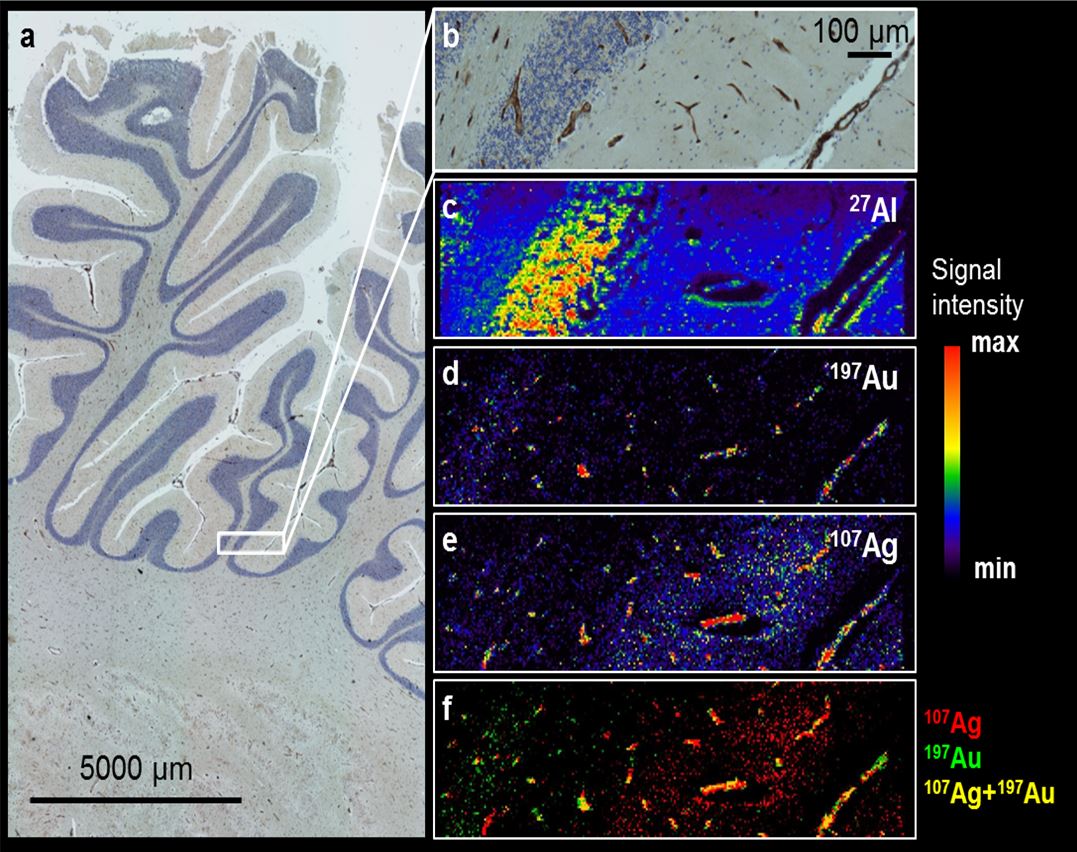
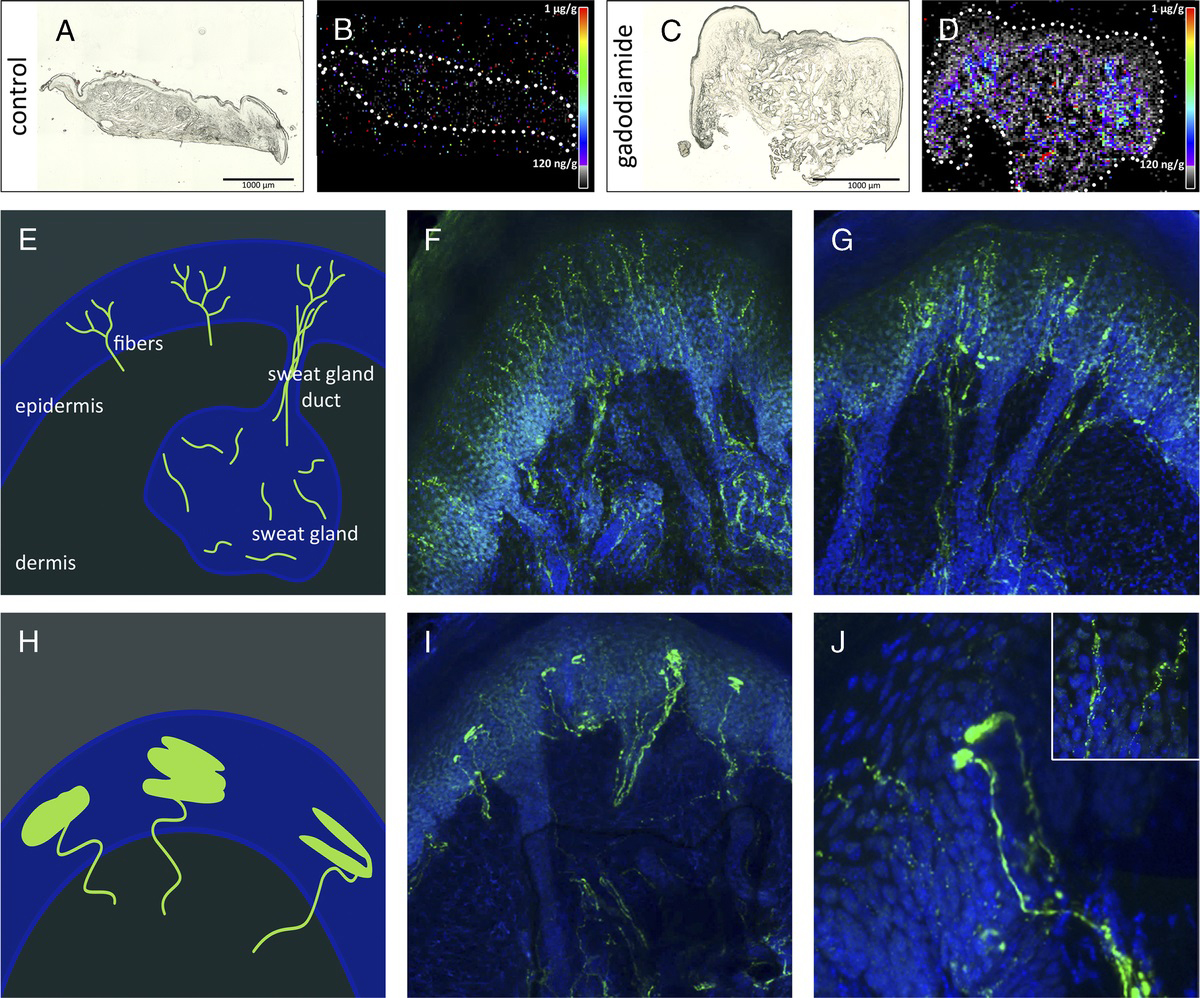
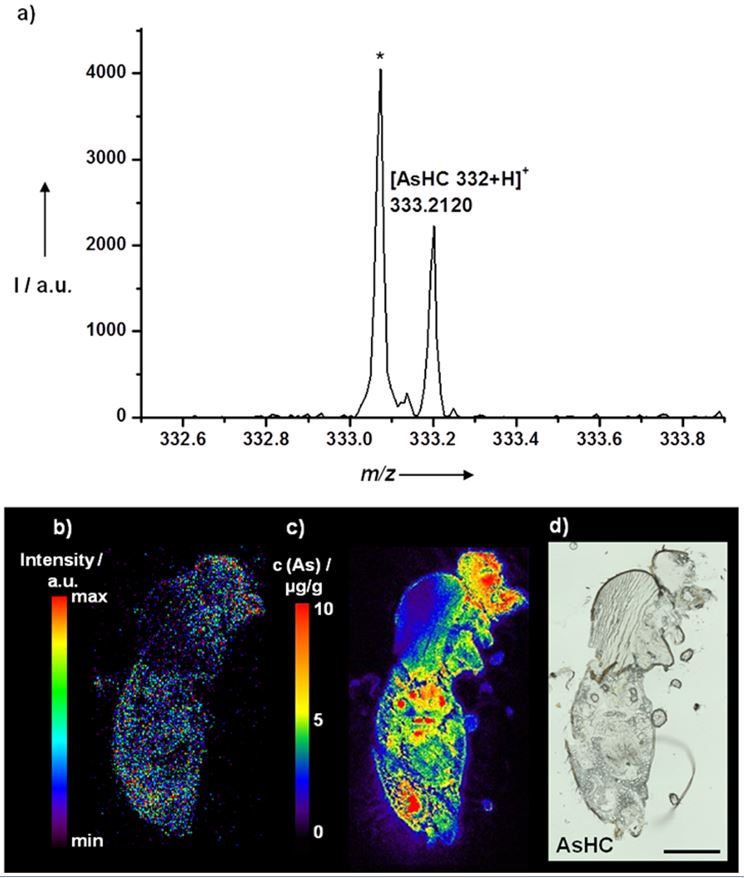
In cooperation with the group of Prof. Uwe Karst (Analytical Chemistry) we are currently studying abnormal deposition of silver (derived from silver-coated endoprostheses, fig. 1) and gadolinium (derived from MRI contrast agents, fig. 2) in human brain tissue and animal studies. Using Drosophila melanogaster we are studying the in-vivo distribution of arsenic-containing lipids that are included in seafood (fig. 3).


Prof. Dr. Julia Bornhorst
Bergische Universität Wuppertal
Lehrstuhl für Lebensmittelchemie
Gaußstr. 20, 42119 Wuppertal, Germany

Prof. Dr. Uwe Karst
University of Münster
Institute of Inorganic and Analytical Chemistry
Corrensstraße 30
48149 Münster, Germany

Prof. Dr. med., ass. jur. Alexander Radbruch
Klinik für Neuroradiologie
Universitätsklinikum Bonn
Venusberg-Campus 1, 53127 Bonn, Germany

Dr. med. vet., Dipl. SVLAS, Henning Richter
Diagnostic Imaging Research Unit (DIRU)
Clinic for Diagnostic Imaging
Department of Clinical Diagnostics and Services
Vetsuisse Faculty
University of Zurich, Switzerland

PD Dr. med. Anne Schänzer
Institute of Neuropathology
University Clinic Giessen and Marburg
Arndtstr. 16, 35392 Giessen, Germany

Prof. Dr. Tanja Schwerdtle,
Institut für Ernährungswissenschaft
Abteilung Lebensmittelchemie
Arthur-Scheunert-Allee 114-116
14558 Nuthetal, OT Bergholz-Rehbrücke, Germany

PD Dr. rer. nat. Christoph van Thriel
Department of Toxicology
Leibniz Research Centre for Working Environment
and Human Factors (IfADo)
Dortmund, Germany
Selected Publications:
- Richter H, Bücker P, Martin LF, Dunker C, Fingerhut S, Xia A, Karol A, Sperling M, Karst U, Radbruch A, Jeibmann A.
Gadolinium Tissue Distribution in a Large-Animal Model after a Single Dose of Gadolinium-based Contrast Agents. Radiology 2021 Sep 21:210553. doi: 10.1148/radiol.2021210553. Online ahead of print. PMID: 34546128
- Bücker P, Richter H, Radbruch A, Sperling M, Brand M, Holling M, Van Marck V, Paulus W, Jeibmann A, Karst U.
Deposition patterns of iatrogenic lanthanum and gadolinium in the human body depend on delivered chemical binding forms. J Trace Elem Med Biol. 2021 Jan;63:126665. doi: 10.1016/j.jtemb.2020.126665. Epub 2020 Oct 24. PMID: 33152670
- Radbruch A, Richter H, Bücker P, Berlandi J, Schänzer A, Deike-Hofmann K, Kleinschnitz C, Schlemmer HP, Forsting M, Paulus W, Martin LF, van Thriel C, Karst U, Jeibmann A.
Is Small Fiber Neuropathy Induced by Gadolinium-Based Contrast Agents? Invest Radiol. 2020 Aug;55(8):473-480. doi: 10.1097/RLI.0000000000000677. PMID: 32604384
- Radbruch A, Richter H, Fingerhut S, Martin LF, Xia A, Henze N, Paulus W, Sperling M, Karst U, Jeibmann A.
Gadolinium Deposition in the Brain in a Large Animal Model: Comparison of Linear and Macrocyclic Gadolinium-Based Contrast Agents. Invest Radiol. 2019 Sep;54(9):531-536. doi: 10.1097/RLI.0000000000000575. PMID: 31261291
- Clases D, Fingerhut S, Jeibmann A, Sperling M, Doble P, Karst U.
LA-ICP-MS/MS improves limits of detection in elemental bioimaging of gadolinium deposition originating from MRI contrast agents in skin and brain tissues. J Trace Elem Med Biol. 2019 Jan;51:212-218. doi: 10.1016/j.jtemb.2018.10.021. Epub 2018 Nov 1. PMID: 30466933
- Fingerhut S, Sperling M, Holling M, Niederstadt T, Allkemper T, Radbruch A, Heindel W, Paulus W, Jeibmann A, Karst U.
Gadolinium-based contrast agents induce gadolinium deposits in cerebral vessel walls, while the neuropil is not affected: an autopsy study. Acta Neuropathol. 2018 Jul;136(1):127-138. doi: 10.1007/s00401-018-1857-4. Epub 2018 May 10. PMID: 29748901
- Fingerhut S, Niehoff AC, Sperling M, Jeibmann A, Paulus W, Niederstadt T, Allkemper T, Heindel W, Holling M, Karst U.
Spatially resolved quantification of gadolinium deposited in the brain of a patient treated with gadolinium-based contrast agents. J Trace Elem Med Biol. 2018 Jan;45:125-130. doi: 10.1016/j.jtemb.2017.10.004. Epub 2017 Oct 13. PMID: 29173468
Special model: Drosophila melanogaster
Drosophila melanogaster as model in neuroscience and neuropathology
We strongly feel that Drosophila represents a fascinating and extremely versatile model for studying pathogenesis of neurological disease as well as for exploring interactions between brain, behavior and environment. This is the reason why we have established a Drosophila lab in our institute building. We are experimentally using the fly in various areas, including neurooncology, neurodegeneration, neurotoxicology and lifestyle neuropathology.
Drosophila melanogaster, as a model organism, offers several advantages, including easy handling, rapid generation time, low cost, and a wide armamentarium of genetic techniques. Many molecular pathways are conserved between invertebrates and humans. Furthermore, Drosophila can be used in neuropharmacological experiments because this organism is amenable to external/food application, inhalation, or injection of substances in a large number of wild type or mutant animals.

Selected publications:
- Berlandi J, Chaouch A, De Jay N, Tegeder I, Thiel K, Shirinian M, Kleinman CL, Jeibmann A, Lasko P, Jabado N, Hasselblatt M
Identification of genes functionally involved in the detrimental effects of mutant histone H3.3-K27M in Drosophila melanogaster. Neuro Oncol. 2019 Jan 23. doi: 10.1093/neuonc/noz021. [Epub ahead of print]
- Tegeder I, Thiel K, Erkek S, Johann PD, Berlandi J, Thatikonda V, Frühwald MC, Kool M, Jeibmann A, Hasselblatt M
Functional relevance of genes predicted to be affected by epigenetic alterations in atypical teratoid/rhabdoid tumors. J Neurooncol. 2019 Jan;141(1):43-55
- Ruland C, Berlandi J, Eikmeier K, Weinert T, Lin FJ, Ambree O, Seggewiss J, Paulus W, Jeibmann A
Decreased Cerebral Irp-1B Limits Impact of Social Isolation in Wildtype and Alzheimer's Disease Modelled in Drosophila melanogaster. Genes Brain Behav. 2018 Jun;17(5):e12451
- Berlandi J, Lin FJ, Ambrée O, Rieger D, Paulus W, Jeibmann A
Swing Boat: Inducing and Recording Locomotor Activity in a Drosophila melanogaster Model of Alzheimer's Disease. Front Behav Neurosci. 2017 Aug 30;11:159
- Niehoff AC, Schulz J, Soltwisch J, Meyer S, Kettling H, Sperling M, Jeibmann A, Dreisewerd K, Francesconi KA, Schwerdtle T, Karst U
Imaging by Elemental and Molecular Mass Spectrometry Reveals the Uptake of an Arsenolipid in the Brain of Drosophila melanogaster. Anal Chem. 2016 May 17;88(10):5258-63
- Niehoff AC, Bauer OB, Kröger S, Fingerhut S, Schulz J, Meyer S, Sperling M, Jeibmann A, Schwerdtle T, Karst U
Quantitative Bioimaging to Investigate the Uptake of Mercury Species in Drosophila melanogaster. Anal Chem. 2015 Oct 20;87(20):10392-6
- Meyer S, Schulz J, Jeibmann A, Taleshi MS, Ebert F, Francesconi KA, Schwerdtle T
Arsenic-containing hydrocarbons are toxic in the in vivo model Drosophila melanogaster. Metallomics. 2014 Nov;6(11):2010-4
- Jeibmann A, Halama K, Witte HT, Kim SN, Eikmeier K, Koos B, Klämbt C, Paulus W
Involvement of CD9 and PDGFR in migration is evolutionarily conserved from Drosophila glia to human glioma. J Neurooncol. 2015 Sep;124(3):373-83
- Kim SN, Jeibmann A, Halama K, Witte HT, Wälte M, Matzat T, Schillers H, Faber C, Senner V, Paulus W, Klämbt C
ECM stiffness regulates glial migration in Drosophila and mammalian glioma models. Development. 2014 Aug;141(16):3233-42
- Jeibmann A, Eikmeier K, Linge A, Kool M, Koos B, Schulz J, Albrecht S, Bartelheim K, Frühwald MC, Pfister SM, Paulus W, Hasselblatt M
Identification of genes involved in the biology of atypical teratoid/rhabdoid tumours using Drosophila melanogaster. Nat Commun. 2014 Jun 3;5:4005
- Witte HT, Jeibmann A, Klämbt C, Paulus W
Modelling glioma growth and invasion in Drosophila melanogaster. Neoplasia. 2009 Sep;11(9):882-8
- Jeibmann A, Paulus W
Drosophila melanogaster as model organism of brain diseases. Int J Mol Sci. 2009 Feb;10(2):407-40
Contact:
Astrid Jeibmann (Email)


