P3: Genomics of severe spermatogenic failure

Frank Tüttelmann, Institute of Reproductive Genetics (Homepage)
Project summary
Non-obstructive azoospermia (NOA) is the clinically most severe form of male infertility, and a testicular biopsy aiming for sperm extraction is the only chance for parenthood. Genetic causes are suspected in the majority of NOA patients, but to date more than 50% remain unexplained.
Within this CRU, we established one of the largest exome datasets of infertile men including >500 NOA patients. These data allowed for the identification of several novel genes in which mutations cause NOA. Examples are TEX11, STAG3, SYCP2, and M1AP. For some of these genes, the molecular role during spermatogenesis has been shown e.g. in model systems. For others, the function of the encoded protein remains elusive, which is the starting point for subsequent analyses. Likewise, variants in these genes may be benign or pathogenic and we will establish proper in vitro test systems to examine patient-specific variants. To identify novel candidate genes, we will significantly increase the number of exome sequenced patients. A complementary fertility screen in Drosophila melanogaster will reveal conserved genes required for spermatogenesis.
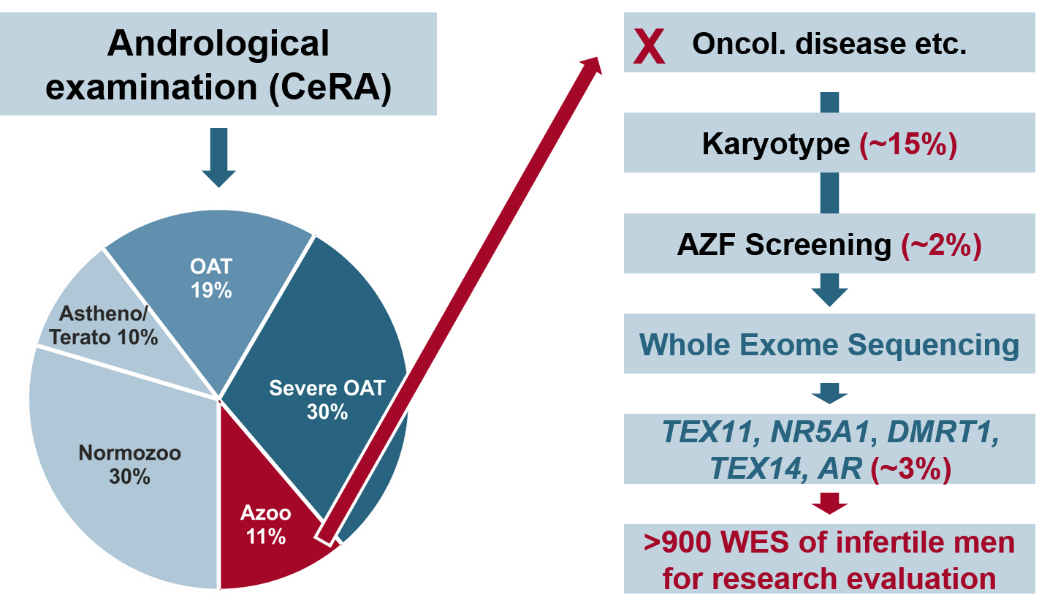
Consequently, we will significantly increase the genetic diagnostic yield in infertile azoospermic men. This will pave the way for well-informed treatment decisions, estimating risks, and better counselling.


