Molecular control of the leaky vascular barrier in sepsis
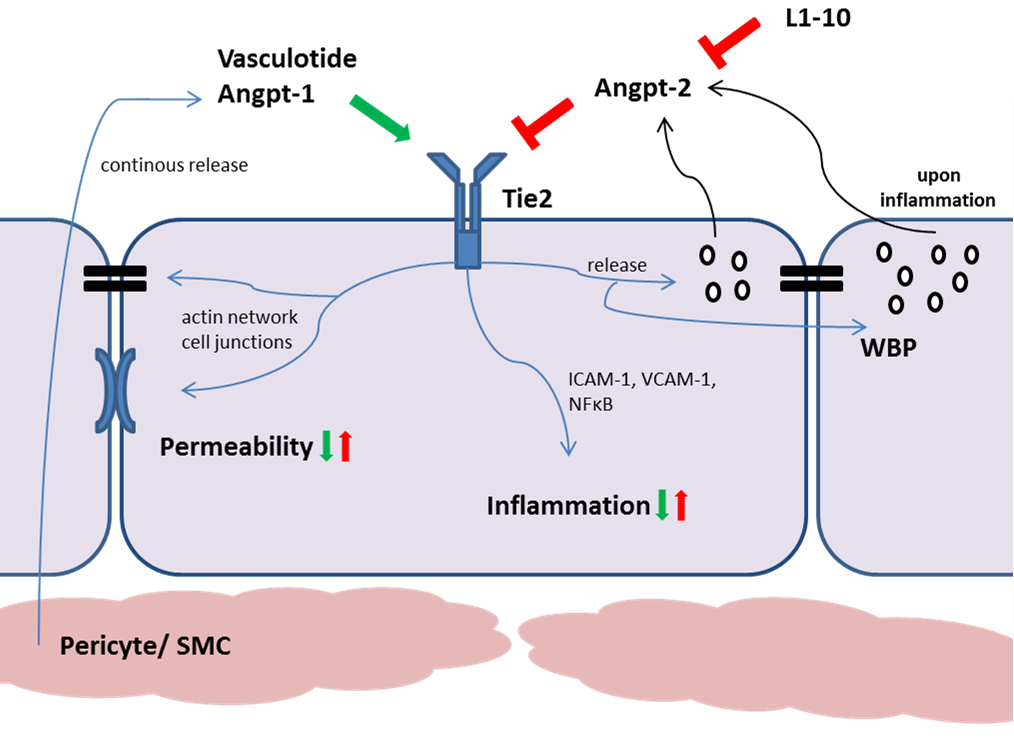
Breakdown of the vascular barrier is a critical event for the development of multiple-organ dysfunction and shock. Over the recent years our group explored the Angiopoietin-Tie2 ligand-receptor pathway, a non-redundant pathway controlling endothelial activity, in sepsis and acute kidney injury. The vascular-associated receptor tyrosine kinases Tie2 and its agonist ligand Angiopoietin-1 (Angpt1) were discovered in the mid-1990s. While early studies in Angpt1-/- and Tie2 -/- knockout mice, which die in utero owing to severe vascular defects, revealed the importance of operational Angpt1-Tie2 signaling for developmental angiogenesis, Angpt1 was subsequently identified as a transdominant anti-permeability factor that protects the vasculature of adult mice from plasma leakage induced by vascular endothelial growth factor and other inflammatory stimuli. In contrast, release of Angiopoietin-2 (Angpt-2) from endothelial Weibel-Pallade bodies disrupts the constitutive Angpt1-Tie2 signaling by preventing Angpt1 from binding to the receptor thereby promoting inflammation and permeability. In several studies including our own, plasma Angiopoietin-2 has been shown to predict the severity and mortality in critically ill patients with sepsis and septic shock. We could show that stimulation of the Tie2 receptor, e.g. by pharmacological Tie2-activation, reduces capillary leakage, multiple-organ dysfunction and mortality in various animal models of experimental sepsis and acute kidney injury. A growing number of translational studies have consistently shown that (maintenance of) operational Angpt-1/Tie2 signaling counteracts – and withdrawal of Tie2 phosphorylation by Angpt2 promotes – (hyper-)permeability through multi-level effects on intracellular signaling, cytoskeleton, and junction-related molecules, culminating in the formation of intercellular gaps between endothelial cells. We could recently show that Angpt2 also mediates breakdown of the endothelial glycocalyx, a protective carbohydrate-rich gel-like mesh of large anionic polymers, that lines the luminal side of the endothelium along the entire vascular tree. Mechanistically, Angpt2 causes heparanase secretion from distinctive cellular storage pools with consecutive enzymatic degradation of the glycocalyx. Own in vivo and in vitro experiments using either recombinant Angpt1 or the synthetic Tie2-peptidomimetic Vasculotide strongly suggest that (super)activation of Tie2 may serve as a potential molecular sealant for leaky vessels.
Tie2 pathways in endothelial cells
Related articles:
Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Lukasz A, Hillgruber C, Oberleithner H, Kusche-Vihrog K, Pavenstädt H, Rovas A, Hesse B, Goerge T, Kümpers P. Cardiovasc Res. 2017 May 1;113(6):671-680. PMID: 28453727
The Synthetic Tie2 Agonist Peptide Vasculotide Protects Renal Vascular Barrier Function In Experimental Acute Kidney Injury. Rübig E, Stypmann J, Van Slyke P, Dumont DJ, Spieker T, Buscher K, Reuter S, Goerge T, Pavenstädt H, Kümpers P. Sci Rep. 2016 Feb 25;6:22111. PMID: 26911791
Nanomechanics of the endothelial glycocalyx in experimental sepsis. Wiesinger A, Peters W, Chappell D, Kentrup D, Reuter S, Pavenstädt H, Oberleithner H, Kümpers P. PLoS One. 2013 Nov 20;8(11):e80905. PMID: 24278345
Mending leaky blood vessels: the angiopoietin-Tie2 pathway in sepsis. David S, Kümpers P, van Slyke P, Parikh SM. J Pharmacol Exp Ther. 2013 Apr;345(1):2-6. PMID: 23378191
Angiopoietin-2 in acute liver failure. Hadem J, Bockmeyer CL, Lukasz A, Pischke S, Schneider AS, Wedemeyer H, Jonigk D, Manns MP, Kümpers P. Crit Care Med. 2012 May;40(5):1499-505. PMID: 22430236
The synthetic tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Kumpers P, Gueler F, David S, Slyke PV, Dumont DJ, Park JK, Bockmeyer CL, Parikh SM, Pavenstadt H, Haller H, Shushakova N. Crit Care. 2011;15(5):R261. PMID: 22040774
Acute administration of recombinant Angiopoietin-1 ameliorates multiple-organ dysfunction syndrome and improves survival in murine sepsis. David S, Park JK, Meurs Mv, Zijlstra JG, Koenecke C, Schrimpf C, Shushakova N, Gueler F, Haller H, Kümpers P. Cytokine. 2011 Aug;55(2):251-9. PMID: 21531574
Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Kümpers P, Hafer C, David S, Hecker H, Lukasz A, Fliser D, Haller H, Kielstein JT, Faulhaber-Walter R. Intensive Care Med. 2010 Mar;36(3):462-70. PMID: 19956923
Bench-to-bedside review: Angiopoietin signalling in critical illness - a future target? van Meurs M, Kümpers P, Ligtenberg JJ, Meertens JH, Molema G, Zijlstra JG. Crit Care. 2009;13(2):207. PMID: 19435476
Time course of angiopoietin-2 release during experimental human endotoxemia and sepsis. Kümpers P, van Meurs M, David S, Molema G, Bijzet J, Lukasz A, Biertz F, Haller H, Zijlstra JG. Crit Care. 2009;13(3):R64. PMID: 19416526
Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Kümpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, Fliser D, Haller H, Kielstein JT. Crit Care. 2008;12(6):R147. PMID: 19025590
Circulating angiopoietin-1 and angiopoietin-2 in critically ill patients: development and clinical application of two new immunoassays. Lukasz A, Hellpap J, Horn R, Kielstein JT, David S, Haller H, Kümpers P. Crit Care. 2008;12(4):R94. PMID: 18664247
